Efficacy and Safety of Natural Apigenin Treatment for Alzheimer's Disease: Focus on In vivo Research Advancements
- PMID: 39665306
- PMCID: PMC12163474
- DOI: 10.2174/1570159X23666241211095018
Efficacy and Safety of Natural Apigenin Treatment for Alzheimer's Disease: Focus on In vivo Research Advancements
Abstract
Background: Alzheimer's Disease (AD) is the most common dementia in clinics. Despite decades of progress in the study of the pathogenesis of AD, clinical treatment strategies for AD remain lacking. Apigenin, a natural flavonoid compound, is present in a variety of food and Chinese herbs and has been proposed to have a wide range of therapeutic effects on dementia.
Objective: To clarify the relevant pharmacological mechanism and therapeutic effect of apigenin on animal models of AD.
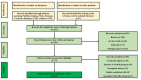
Methods: Computer-based searches of the PubMed, Cochrane Library, Embase, and Web of Science databases were used to identify preclinical literature on the use of apigenin for treating AD. All databases were searched from their respective inception dates until June 2023. The meta-analysis was performed with Review manager 5.4.1 and STATA 17.0.
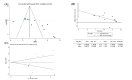
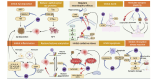
Results: Thirteen studies were eventually enrolled, which included 736 animals in total. Meta-analysis showed that apigenin had a positive effect on AD. Compared to controls, apigenin treatment reduced escape latency, increased the percentage of time spent in the target quadrant and the number of plateaus traversed; apigenin was effective in reducing nuclear factor kappa-B (NF-κB) p65 levels; apigenin effectively increased antioxidant molecules SOD and GSH-px and decreased oxidative index MDA; for ERK/CREB/BDNF pathway, apigenin effectively increased BDNF and pCREB molecules; additionally, apigenin effectively decreased caspase3 levels and the number of apoptotic cells in the hippocampus.
Conclusion: The results show some efficacy of apigenin in the treatment of AD models. However, further clinical studies are needed to confirm the clinical efficacy of apigenin.
Keywords: Alzheimer's disease; apigenin; apigenin treatment.; meta-analysis; pharmacological mechanism; systematic review.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures











Similar articles
-
Beneficial effects of apigenin on the transgenic Drosophila model of Alzheimer's disease.Chem Biol Interact. 2022 Oct 1;366:110120. doi: 10.1016/j.cbi.2022.110120. Epub 2022 Aug 24. Chem Biol Interact. 2022. PMID: 36027948
-
Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-canonical NF-κB Pathway In Vivo and In Vitro.Inflammation. 2020 Oct;43(5):1634-1648. doi: 10.1007/s10753-020-01238-5. Inflammation. 2020. PMID: 32458347
-
Pharmacological basis for application of scutellarin in Alzheimer's disease: Antioxidation and antiapoptosis.Mol Med Rep. 2018 Nov;18(5):4289-4296. doi: 10.3892/mmr.2018.9482. Epub 2018 Sep 13. Mol Med Rep. 2018. PMID: 30221730 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Verum- versus Sham-Acupuncture on Alzheimer's Disease (AD) in Animal Models: A Preclinical Systematic Review and Meta-Analysis.Biomed Res Int. 2020 Mar 31;2020:5901573. doi: 10.1155/2020/5901573. eCollection 2020. Biomed Res Int. 2020. PMID: 32337259 Free PMC article.
References
-
- Benmelouka A.Y., Ouerdane Y., Outani O., Alnasser Y.T., Alghamdi B.S., Perveen A., Ashraf G.M., Ebada M.A. Alzheimer’s disease-related psychosis: An overview of clinical manifestations, pathogenesis, and current treatment. Curr. Alzheimer Res. 2022;19(4):285–301. doi: 10.2174/1567205019666220418151914. - DOI - PubMed
-
- Yang A., Wu J., Chen Y., Shen R., Kou X. Study on multi-target synergistic treatment of alzheimer’s disease based on metal chelators. Curr. Drug Targets. 2022;24(2):131–150. - PubMed
-
- Shah H., Albanese E., Duggan C., Rudan I., Langa K.M., Carrillo M.C., Chan K.Y., Joanette Y., Prince M., Rossor M., Saxena S., Snyder H.M., Sperling R., Varghese M., Wang H., Wortmann M., Dua T. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15(12):1285–1294. doi: 10.1016/S1474-4422(16)30235-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

