Discovery of VU6024578/BI02982816: An mGlu1 Positive Allosteric Modulator with Efficacy in Preclinical Antipsychotic and Cognition Models
- PMID: 39665415
- PMCID: PMC11684029
- DOI: 10.1021/acs.jmedchem.4c02554
Discovery of VU6024578/BI02982816: An mGlu1 Positive Allosteric Modulator with Efficacy in Preclinical Antipsychotic and Cognition Models
Abstract
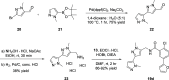
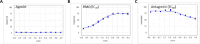
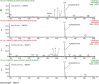
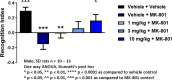
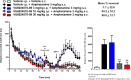
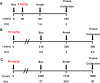
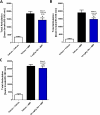
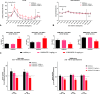
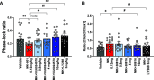
Herein, we report progress toward a metabotropic glutamate receptor subtype 1 (mGlu1) positive allosteric modulator (PAM) clinical candidate and the discovery of VU6024578/BI02982816. From a weak high-throughput screening hit (VU0538160, EC50 > 10 μM, 71% Glumax), optimization efforts improved functional potency over 185-fold to deliver the selective (inactive on mGlu2-5,7,8) and CNS penetrant (rat Kp = 0.99, Kp,uu = 0.82; MDCK-MDR1 ER = 1.7, Papp = 73 × 10-6 cm/s) mGlu1 PAM (VU6024578/BI02982816, EC50 = 54 nM, 83% Glumax). An excellent rat pharmacokinetic profile allowed the evaluation of VU6024578/BI02982816 in both amphetamine-induced hyperlocomotion (minimum effective dose (MED) = 3 mg/kg, p.o.) and MK-801 induced disruptions of novel object recognition (MED = 10 mg/kg p.o.), thus providing efficacy in preclinical models of psychosis and cognition. However, unanticipated AEs in dog prevented further consideration as a candidate. Thus, VU6024578/BI02982816 can serve as a best-in-class in vivo rodent tool to study selective mGlu1 activation.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors were previously developing mGlu1 PAMs in collaboration with Boehringer Ingelheim.
Figures



References
-
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauthier S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; Potter W. Z.; Paul S. M. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997, 54 (4), 465–473. 10.1001/archneur.1997.00550160091022. - DOI - PubMed
-
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dubé S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008, 165, 1033–1039. 10.1176/appi.ajp.2008.06091591. - DOI - PubMed
-
- Kaul I.; Sawchak S.; Correll C. U.; Kakar R.; Breier A.; Zhu H.; Miller A. C.; Paul S. M.; Brannan S. K. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline–trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 2024, 403, 160–170. 10.1016/S0140-6736(23)02190-6. - DOI - PubMed
-
- Krystal J. H.; Kane J. M.; Correll C. U.; Walling D. P.; Leoni M.; Duvvuri S.; Patel S.; Chang I.; Iredale P.; Frohlich L.; Versavel S.; Perry P.; Sanchez R.; Renger J. Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part, randomised, double-blind, placebo-controlled, phase 1b trial. Lancet 2022, 400, 2210–2220. 10.1016/S0140-6736(22)01990-0. - DOI - PubMed
-
- Foster D. J.; Wilson J. M.; Remke D. H.; Mahmood M. S.; Uddin M. J.; Wess J.; Patel S.; Marnett L. J.; Niswender C. M.; Jones C. K.; Xiang Z.; Lindsley C. W.; Rook J. M.; Conn P. J. M4 activation reduces striatal dopamine release and has antipsychotic-like effects via a CB2 cannabinoid receptor-dependent mechanism. Neuron 2016, 91, 1244–1252. 10.1016/j.neuron.2016.08.017. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

