Correlation Between Electronic Patient-Reported Outcomes and Biological Markers of Key Parameters in Acute Radiation Cystitis Among Patients With Prostate Cancer (RABBIO): Prospective Observational Study
- PMID: 39665773
- PMCID: PMC11656992
- DOI: 10.2196/48225
Correlation Between Electronic Patient-Reported Outcomes and Biological Markers of Key Parameters in Acute Radiation Cystitis Among Patients With Prostate Cancer (RABBIO): Prospective Observational Study
Abstract
Background: Despite advances in radiation techniques, radiation cystitis (RC) remains a significant cause of morbidity from pelvic radiotherapy, which may affect patients' quality of life (QoL). The pathophysiology of RC is not well understood, which limits the development of effective treatments.
Objective: The Radiotoxicity Bladder Biomarkers study aims to investigate the correlation between blood and urinary biomarkers and the intensity of acute RC symptoms and QoL in patients undergoing localized prostate cancer radiotherapy.
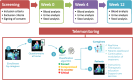
Methods: This study included patients with low- or intermediate-risk localized prostate cancer who were eligible for localized radiotherapy. Blood and urinary biomarkers were analyzed before radiotherapy was initiated and at weeks 4 and 12 of radiation therapy. Patients completed questionnaires related to RC symptoms and QoL (International Prostate Symptom Score and Functional Assessment of Cancer Therapy-Prostate [FACT-P]) using a digital remote monitoring platform. The information was processed by means of an algorithm, which classified patients according to the severity of symptoms and adverse events reported. Levels of blood and urinary biomarkers were tested with the severity of acute RC symptoms and patient-reported QoL.
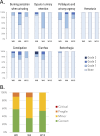
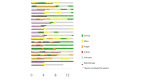
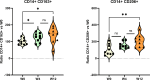
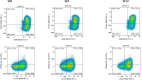
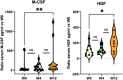
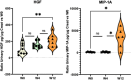
Results: A total of 401 adverse events questionnaires were collected over the duration of this study from 20 patients. The most frequently reported adverse events at week 4 were pollakiuria, constipation, and diarrhea. In comparison with baseline, the mean FACT-P score decreased at week 4. A significant increase in the proportion of M2 phenotype cells (CD206+, CD163+, CD204+) at W12 compared to W0 was observed. An increase in serum and urine levels of macrophage colony-stimulating factor (M-CSF), hepatocyte growth factor, and macrophagic inflammatory protein was observed at week 12 compared to baseline levels. Baseline serum and urine M-CSF concentrations showed a significant negative correlation with FACT-P scores at weeks 4 and 12 (r=-0.65, P=.04, and r=-0.76, P=.02, respectively).
Conclusions: The Radiotoxicity Bladder Biomarkers study is the first to explore the overexpression of inflammatory proteins in blood and urine of patients with symptoms of acute RC. These preliminary findings suggest that serum and urine levels of hepatocyte growth factor, M-CSF, and macrophagic inflammatory protein, as well as macrophage polarization, are mobilized after prostate radiotherapy. The elevated M-CSF levels in serum and urine at baseline were associated with the deterioration of QoL during radiotherapy. The results of this study may help to develop mitigation strategies to limit radiation damage to the bladder.
Keywords: acute radiation cystitis; biomarkers; e-PRO; electronic patient-reported outcome; prostate cancer; quality of life.
© Carole Helissey, Sophie Cavallero, Nathalie Guitard, Hélène Thery, Charles Parnot, Antoine Schernberg, Imen Aissa, Florent Raffin, Christine Le Coz, Stanislas Mondot, Christos Christopoulos, Karim Malek, Emmanuelle Malaurie, Pierre Blanchard, Cyrus Chargari, Sabine Francois. Originally published in JMIR Cancer (https://cancer.jmir.org).
Conflict of interest statement
Figures
References
-
- Le cancer de la prostate - les cancers les plus fréquents. Intitut National du Cancer. [9-11-2024]. https://www.e-cancer.fr/Professionnels-de-sante/Les-chiffres-du-cancer-e... URL. Accessed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

