Eptinezumab treatment was associated with longer interictal headache/migraine periods which corresponded to greater improvements in patient-reported quality of life measures
- PMID: 39666143
- PMCID: PMC11638385
- DOI: 10.1007/s00415-024-12809-z
Eptinezumab treatment was associated with longer interictal headache/migraine periods which corresponded to greater improvements in patient-reported quality of life measures
Abstract
Introduction: Longer periods between headache episodes (interictal periods) may provide greater time for the nervous system to reset from a previous episode, potentially improving disease status and health-related quality of life. This post hoc analysis evaluated this hypothesis by associating patients' longest interictal periods with improvements in patient-reported outcomes.
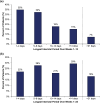
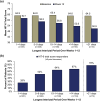
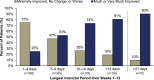
Methods: PROMISE-2 (NCT02974153) was a double-blind, placebo-controlled study evaluating eptinezumab for preventive treatment of chronic migraine (N = 1072). Daily electronic diary data from Weeks 1-12 and Weeks 1-24 were used to identify interictal periods, defined as days between headache episodes. For each patient, the longest interictal period within these intervals was identified and categorized (1-4, 5-9, 10-14, > 14, and > 21 days). For each category, the following patient-reported outcomes were assessed: 6-item Headache Impact Test (HIT-6), Patient Global Impression of Change (PGIC), and patient-identified most bothersome symptom (PI-MBS).
Results: Excluding interictal periods with > 10% missing data (resulting in 1010 patients with sufficient data), the mean (SD) of longest interictal periods over Weeks 1-12 was 9.4 (11.0) days. A ≥6-point HIT-6 reduction was observed in 78% (56/72) vs 26% (91/351) of patients with a > 21-day vs 1-4-day longest interictal period, respectively; much or very much improvement per PGIC was reported in 90% (65/72) vs 25% (87/348), respectively, and per PI-MBS was reported in 88% (63/72) vs 26% (92/348), respectively. Similar results were observed for Weeks 1-24.
Conclusion: Longer interictal periods were associated with more patients indicating positive changes in headache-related life impact, disease status, and symptomology.
Trial registration: ClinicalTrials.gov (identifier: NCT02974153; registered: 2016-11-23).
Keywords: Chronic migraine; Eptinezumab; Patient-reported outcomes; Preventive migraine treatment; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: SJT reports research funding from AbbVie, Aeon, Amgen, Annovis, Axsome, Cassava, Cognition, Eli Lilly, Inhibikase, Ipsen, Lundbeck, Merz, Neurolief, Pfizer, PrecisionMed, Revance, Scilex, Suven, and UCB. He served as a consultant and/or on advisory boards (honoraria) from AbbVie, Aeon, Alphasights, Amgen, Aruene/eNeura, Atheneum, Axsome Therapeutics, Becker Pharmaceutical Consulting, ClearView Healthcare Partners, ClickTherapeutics, CoolTech, CRG, Decision Resources, Defined Health, DRG, Eli Lilly, ExpertConnect, FCB Health, Fenix, GLG, Guidepoint Global, Health Advances, Health Science Communications, HMP Communications, Impel, Initiator Pharma, InteractiveForums, Keyquest, Ki Health Partners, Krog and Partners, Lundbeck, M3 Global Research, Magellan Health, Magnolia Innovation, Miravo Healthcare, MJH Holdings, Neurofront Therapeutics, Neurolief, Nocira, Novartis, P Value Communications, Pain Insights, Palion Medical, Perfood, Pfizer, Pulmatrix, Putnam Associates, Rehaler, SAI MedPartners, Satsuma, Scilex, Slingshot Insights, Spherix Global Insights, Strategy Inc, Synapse Medical Communication, System Analytic, Taylor and Francis, Tegus, Teva, Theranica, Tonix, Trinity Partners, Unity HA, Vial, and XOC. He received salary from Dartmouth-Hitchcock Medical Center, Thomas Jefferson University, and Ki Health Partners. He serves on the speakers' bureau for AbbVie, Eli Lilly, Pfizer, Scilex, and Teva. He received CME honoraria from the American Academy of Neurology, American Headache Society, Annenberg Center for Health Sciences, Catamount Medical Education, Diamond Headache Clinic, Forefront Collaborative, Haymarket Medical Education, HMP Global, Medical Education Speakers Network, Medical Learning Institute Peerview, Migraine Association of Ireland, Miller Medical Education, National Association for Continuing Education, North American Center for CME, The Ohio State University, Physicians’ Education Resource, PlatformQ Education, Primed, Vindico Medical Education, and WebMD/Medscape. MLD is an advisory board member for Amgen, Assertio, Eli Lilly, Lundbeck, Promius Pharma, Supernus Pharmaceuticals, Teva, and Upsher‐Smith Laboratories; a consultant for Amgen, Eli Lilly, Lundbeck, Promius Pharma, and Teva; and speaker's bureau for Amgen, Assertio, Eli Lilly, Supernus Pharmaceuticals, and Teva. DA and DF are full-time employees of Lundbeck LLC. JH is an employee of Pacific Northwest Statistical Consulting, Inc., a company that received funding from Lundbeck LLC for time spent conducting this research. RC was an employee of Lundbeck LLC for the development and conduct of PROMISE-2 and currently consults with Lundbeck and is a part time employee at Axon. Ethical approval: This study was approved by the independent ethics committee or institutional review board at each study site. All clinical work was conducted in compliance with current good clinical practices, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, local regulatory requirements, and the principles of the Declaration of Helsinki. Consent to participate/publish: All patients enrolled in the study provided written informed consent before any study procedures.
Figures

References
-
- Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, Ashina M, van den Maagdenberg A, Dodick DW (2022) Migraine. Nat Rev Dis Primers 8:2. 10.1038/s41572-021-00328-4 - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38: 1–211. 10.1177/0333102417738202 - PubMed
-
- Bigal ME, Lipton RB (2006) When migraine progresses: transformed or chronic migraine. Expert Rev Neurother 6:297–306. 10.1586/14737175.6.3.297 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

