Clinical tolerance but no protective efficacy in a placebo-controlled trial of repeated controlled schistosome infection
- PMID: 39666392
- PMCID: PMC11827845
- DOI: 10.1172/JCI185422
Clinical tolerance but no protective efficacy in a placebo-controlled trial of repeated controlled schistosome infection
Abstract
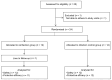
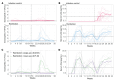
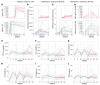
BACKGROUNDPartial protective immunity to schistosomiasis develops over time, following repeated praziquantel (PZQ) treatment. Moreover, animals develop protective immunity after repeated immunization with irradiated cercariae. Here, we evaluated the development of natural immunity through consecutive exposure-treatment cycles with Schistosoma mansoni in healthy, Schistosoma-naive participants using single-sex, controlled human S. mansoni infection.METHODSTwenty-four participants were randomized in a double-blinded (1:1) manner to either the reinfection group, which received 3 exposures (weeks 0, 9, and 18) to 20 male cercariae, or to the infection control group, which received 2 mock exposures with water (weeks 0 and 9) prior to cercariae exposure (week 18). Participants were treated with PZQ (or placebo) at weeks 8, 17, and 30. Attack rates (ARs) after the final exposure (weeks 19-30) using serum circulating anodic antigen (CAA) positivity were compared between groups. Adverse events (AEs) were collected for safety.RESULTSTwenty-three participants completed the follow-up. No protective efficacy was observed, given an 82% (9 of 11) AR after the final exposure in the reinfection group and 92% (11 of 12) in the infection control group (protective efficacy 11%; 95% CI -24% to 35%; P = 0.5). Related AEs were higher after the first infection (45%) compared with the second (27%) and third (28%) infections. Severe acute schistosomiasis was observed after the first infections only (2 of 12 in the reinfection group and 2 of 12 in the infection control group).CONCLUSIONRepeated Schistosoma exposure and treatment cycles resulted in apparent clinical tolerance, with fewer symptoms reported following subsequent infections, but did not result in protection against reinfection.TRIAL REGISTRATIONClinicalTrials.gov NCT05085470.FUNDINGEuropean Research Council (ERC) Starting Grant (no. 101075876).
Keywords: Infectious disease; Parasitology.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

