Immunogenicity and safety following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine with Matrix-MTM adjuvant (NVX-CoV2373) versus a primary series in people living with and without HIV-1 infection in South Africa: A randomized crossover phase 2a/2b trial
- PMID: 39666396
- PMCID: PMC11789733
- DOI: 10.1080/21645515.2024.2425147
Immunogenicity and safety following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine with Matrix-MTM adjuvant (NVX-CoV2373) versus a primary series in people living with and without HIV-1 infection in South Africa: A randomized crossover phase 2a/2b trial
Abstract
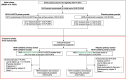
COVID-19 remains a global public health issue and an improved understanding of vaccine performance in immunocompromised individuals, including people living with HIV (PLWH), is needed. Initial data from the present study's pre-crossover/booster phase were previously reported. This phase 2a/b clinical trial in South Africa (2019nCoV-501/NCT04533399) revisits 1:1 randomly assigned HIV-negative adults (18-84 years) and medically stable PLWH (18-64 years) who previously received two NVX-CoV2373 doses (5 μg recombinant Spike protein with 50 μg Matrix-M™ adjuvant) or placebo. During the 6-month blinded crossover/booster phase, NVX-CoV2373 recipients could receive a single NVX-CoV2373 booster dose and placebo recipients a 2-dose NVX-CoV2373 primary series. NVX-CoV2373 safety and immunogenicity were assessed according to prior SARS-CoV-2 infection and HIV status. Post-crossover, 1900/3793 NVX-CoV2373 recipients were assigned another dose, and 1893/3793 placebo recipients were assigned NVX-CoV2373 primary series. Approximately 56% of the participants were SARS-CoV-2-seropositive ("seropositive") at crossover (6% PLWH). In seropositive participants (HIV-negative and PLWH), booster-dose anti-spike IgG, MN50 and hACE2 inhibition responses increased to similar levels, exceeding those in seronegative participants. In primary-series and booster cohorts, seronegative PLWH showed higher neutralizing responses (4.9- to 5.5-fold, respectively) versus peak pre-crossover primary-series responses. The safety profile was similar among the pre-crossover/booster phase groups; solicited and unsolicited adverse events were infrequent in all groups. A single NVX-CoV2373 booster dose substantially increased antibodies. All baseline seropositive participants showed higher immune responses than seronegative participants. These findings support use of NVX-CoV2373, including in immunocompromised individuals.
Keywords: Novavax, Inc.; and the Coalition for Epidemic Preparedness Innovations; the Bill & Melinda Gates Foundation.
Conflict of interest statement
SAM reports receiving grant support, paid to his institution, from BMGF, Novavax, Pfizer, GlaxoSmithKline, Minervax, MERK, providence, Gritstone, and ImmunityBio. QB reports receiving grant support, paid to his institution, from Wits Health Consortium, Bill & Melinda Gates Foundation, South African Medical Research Council, AstraZeneca Pharmaceuticals, Sinovac, Johnson & Johnson, and Pfizer. LFF reports receiving financial support from Novavax for trial procedures. LF reports receiving fees as a contractor and being a paid employee and stock shareholder of Novavax, Inc. GMG reports receiving fees as a consultant and being a paid employee and stock shareholder of Novavax, Inc. All authors who are or used to be employees of Novavax, Inc. may hold stock of Novavax, Inc. All other authors declare no competing interests.
Figures

References
-
- Centers for Disease Control and Prevention . COVID data tracker. 2022. [accessed 2022 Nov 10]. https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
