Hypertonic saline nasal irrigation and gargling for suspected or confirmed COVID-19: Pragmatic randomised controlled trial (ELVIS COVID-19)
- PMID: 39666578
- PMCID: PMC11636951
- DOI: 10.7189/jogh.14.05027
Hypertonic saline nasal irrigation and gargling for suspected or confirmed COVID-19: Pragmatic randomised controlled trial (ELVIS COVID-19)
Abstract
Background: In a previous pilot randomised controlled trial conducted on UK adults, we found that hypertonic saline nasal irrigation and gargling (HSNIG) reduced common cold symptoms, the need for over-the-counter medications, viral shedding, and the duration and transmission of the illness. It is unclear whether HSNIG improves outcomes of the coronavirus disease 2019 (COVID-19). Hypertonic saline can be prepared and HSNIG performed at home, making it a safe and scalable intervention, particularly well-suited for low- and middle-income countries.
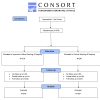
Methods: We conducted a pragmatic randomised controlled trial in Pakistan on adults with suspected or confirmed COVID-19, initially within 48 hours of symptom onset, later extended to within five days due to recruitment challenges. Participants were randomised to one of two groups: the intervention group received instructions on preparing a 2.6% hypertonic saline solution for HSNIG, while the control group was instructed on performing ablution for Muslim prayers (wudu), which involves nasal washing and gargling with tap water. Our primary outcome was the time to symptom resolution, measured by two consecutive days of scoring zero on relevant questions from the validated, self-reported, adapted short form of the Wisconsin Upper Respiratory Symptom Survey (WURSS-24). Secondary outcomes included the severity of all symptoms, the severity and time to resolution of individual symptoms, health care contacts (GP/physician, emergency contacts), hospital attendance (and length of stay if admitted), over-the-counter (OTC) medication (frequency and cost), and transmission to household contacts. The analysis was conducted on an intention-to-treat basis. Logistic regression was used to calculate adjusted odds ratios (aORs) of improvement and Cox regression to calculate adjusted hazard ratios (aHRs) for the time to improvement with accompanying 95% confidence intervals (CIs).
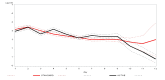
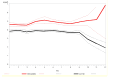
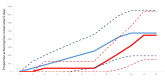
Results: We randomised 576 people: 279 to the HSNIG group and 297 to the control group. Among those, 10 out of 279 (3.6%) in the HSNIG had symptom resolution, compared with 11 out of 297 (3.7%) in the control group (aOR = 1.20, 95% CI = 0.46- 3.22). The time-to-event analysis also showed no significant benefit (aHR = 1.23, 95% CI = 0.51-2.97). Excluding the 127 participants with no data on the primary outcome (who did not complete the study), 10 out of 222 (4.5%) in the HSNIG group had symptom resolution, compared to 11 out of 227 (4.8%) in the control group.
Conclusions: HSNIG was not effective for individuals with suspected or confirmed COVID-19 who began the intervention within five days of symptoms onset and therefore cannot be recommended for use. Further investigation is needed for interventions started within 48 hours of illness onset.
Registration: ClinicalTrials.gov (NCT05104372).
Copyright © 2024 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose the following activities and/or relationships: AS was a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group and is a member of its Standing Committee on Pandemics. He was also a member of the UK Government’s New and Emerging Virus Threats Advisory Group (NERVTAG) Risk Stratification Subgroup. All roles are unremunerated. All other authors report no relevant conflicts of interest.
Figures
References
-
- Worldometer. Coronavirus. 2024. Available: https://www.worldometers.info/coronavirus/. Accessed: 4 January 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
