Phosphorus-independent role of FGF23 in erythropoiesis and iron homeostasis
- PMID: 39666728
- PMCID: PMC11637385
- DOI: 10.1371/journal.pone.0315228
Phosphorus-independent role of FGF23 in erythropoiesis and iron homeostasis
Abstract
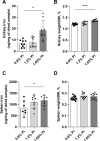
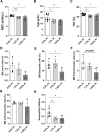
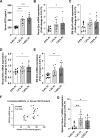
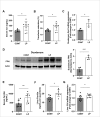
A number of studies have reported an association between phosphorus, red blood cell (RBC) production, and iron metabolism. However, it is difficult to distinguish whether the effect of phosphorus is direct or through the actions of FGF23, and it is not clear whether phosphorus is positively or negatively associated with RBC production. In the present study, we investigated the effects of a) increased phosphorus load and b) phosphorus deficiency on erythropoiesis and iron metabolism in association with FGF23. Mice were fed either a 1.2% or 1.65% phosphorus diet and compared to mice fed a control diet containing 0.6% of phosphorus. Moreover, we used two mouse models of hypophosphatemia-induced either by dietary intervention in the form of a low phosphorus (LP) diet (0.02% of Pi) or genetically in a mouse model of X-linked hypophosphatemia (XLH)-that had opposite FGF23 levels. Phosphorus supplementation appropriately increased FGF23 levels leading to excretion of excess phosphorus and normalization of serum phosphorus levels. We also found that a phosphorus-rich diet results in inflammation-induced hypoferremia associated with reduced iron export leading to tissue iron overload. Moreover, high phosphorus intake results in ineffective erythropoiesis caused by decreased production (decreased RBCs, hemoglobin, hematocrit, and erythroid progenitors in the bone marrow) and increased destruction of RBCs, leading to anemia despite increased EPO secretion. These complications occur through the actions of elevated FGF23 in the presence of normophosphatemia. Our data also show that LP diet induces a decrease in the serum concentrations of phosphorus and FGF23, resulting in increased RBC counts, hemoglobin concentration, and hematocrit compared to mice fed normal diet. Moreover, serum iron and transferrin saturation were increased and positively correlated with serum ferritin, liver ferritin protein and mRNA expression in mice fed LP diet. However, hyp mice, the murine model of XLH, exhibit hypophosphatemia and high serum FGF23 levels, along with low number of circulating RBCs, hemoglobin, and hematocrit compared to wild-type mice. In the bone marrow, hyp mice showed reduced number of erythroid progenitors and formed significantly less BFU-E colonies compared to control mice. Serum iron levels and transferrin saturation were also decreased in hyp mice in comparison to control mice. Taken together, our data show that FGF23 acts independent of phosphorus levels to regulate erythropoiesis and iron homeostasis.
Copyright: © 2024 Park et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

