Semisynthetic Glycoconjugate Vaccine Lead against Klebsiella pneumoniae Serotype O2afg Induces Functional Antibodies and Reduces the Burden of Acute Pneumonia
- PMID: 39666976
- PMCID: PMC11673581
- DOI: 10.1021/jacs.4c13972
Semisynthetic Glycoconjugate Vaccine Lead against Klebsiella pneumoniae Serotype O2afg Induces Functional Antibodies and Reduces the Burden of Acute Pneumonia
Abstract
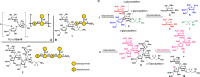
Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) bacteria are a serious global health concern due to their drug-resistance to nearly all available antibiotics, fast spread, and high mortality rate. O2afg is a major CR-Kp serotype in the sequence type 258 group (KPST258) that is weakly immunogenic in humans. Here, we describe the creation and evaluation of semisynthetic O2afg glycoconjugate vaccine leads containing one and two repeating units of the polysaccharide epitope that covers the surface of the bacteria conjugated to the carrier protein CRM197. The semisynthetic glycoconjugate containing two repeating units induced functional IgG antibodies in rabbits with opsonophagocytic killing activity and enhanced complement activation and complement-mediated killing of CR-Kp. Passive immunization reduced the burden of acute pneumonia in mice and may represent an alternative to antimicrobial therapy. The semisynthetic glycoconjugate vaccine lead against CR-Kp expressing O2afg antigen is awaiting preclinical development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Global Report on Infection Prevention and Control; World Health Organization, 2022.
-
- Lou T.; Du X.; Zhang P.; Shi Q.; Han X.; Lan P.; Yan R.; Hu H.; Wang Y.; Wu X.; et al. Risk factors for infection and mortality caused by carbapenem-resistant Klebsiella pneumoniae: A large multicentre case-control and cohort study. J. Infect. 2022, 84 (5), 637–647. 10.1016/j.jinf.2022.03.010. - DOI - PubMed
-
- Antibiotic resistance threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.); National Center for Emerging Zoonotic and Infectious Diseases (U.S.). Division of Healthcare Quality Promotion. Antibiotic Resistance Coordination and Strategy Unit., 2019.
-
- Marsh J. W.; Mustapha M. M.; Griffith M. P.; Evans D. R.; Ezeonwuka C.; Pasculle A. W.; Shutt K. A.; Sundermann A.; Ayres A. M.; Shields R. K.; et al. Evolution of Outbreak-Causing Carbapenem-Resistant Klebsiella pneumoniae ST258 at a Tertiary Care Hospital over 8 Years. mBio 2019, 10 (5), e0194510.1128/mbio.01945-19. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

