The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation
- PMID: 39666979
- PMCID: PMC12132800
- DOI: 10.1021/acs.chemrev.3c00755
The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation
Abstract
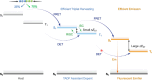
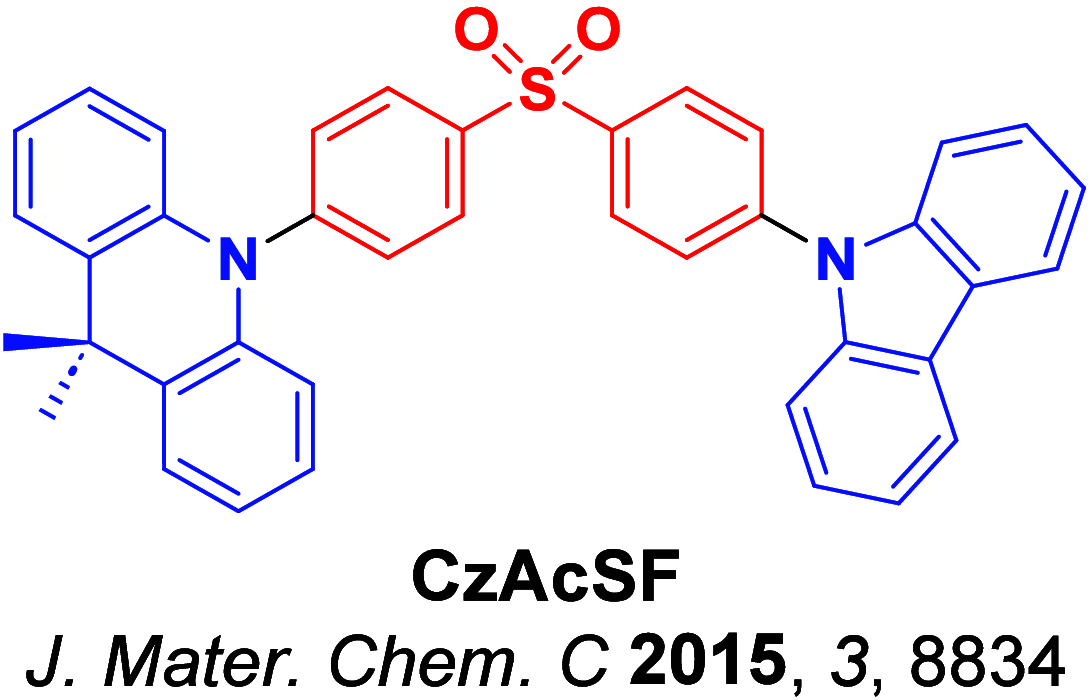
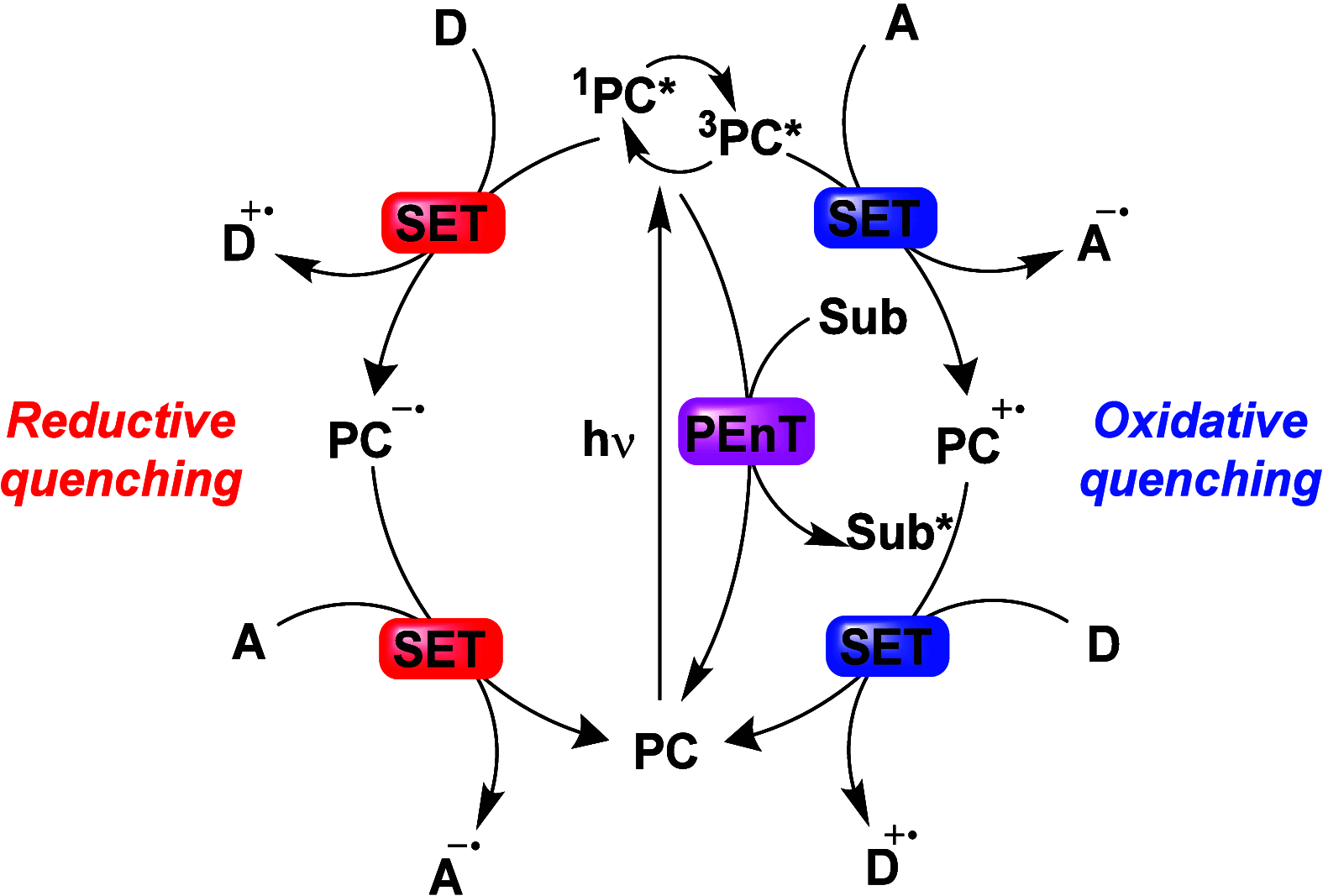
Since the seminal report by Adachi and co-workers in 2012, there has been a veritable explosion of interest in the design of thermally activated delayed fluorescence (TADF) compounds, particularly as emitters for organic light-emitting diodes (OLEDs). With rapid advancements and innovation in materials design, the efficiencies of TADF OLEDs for each of the primary color points as well as for white devices now rival those of state-of-the-art phosphorescent emitters. Beyond electroluminescent devices, TADF compounds have also found increasing utility and applications in numerous related fields, from photocatalysis, to sensing, to imaging and beyond. Following from our previous review in 2017 ( Adv. Mater. 2017, 1605444), we here comprehensively document subsequent advances made in TADF materials design and their uses from 2017-2022. Correlations highlighted between structure and properties as well as detailed comparisons and analyses should assist future TADF materials development. The necessarily broadened breadth and scope of this review attests to the bustling activity in this field. We note that the rapidly expanding and accelerating research activity in TADF material development is indicative of a field that has reached adolescence, with an exciting maturity still yet to come.
Figures
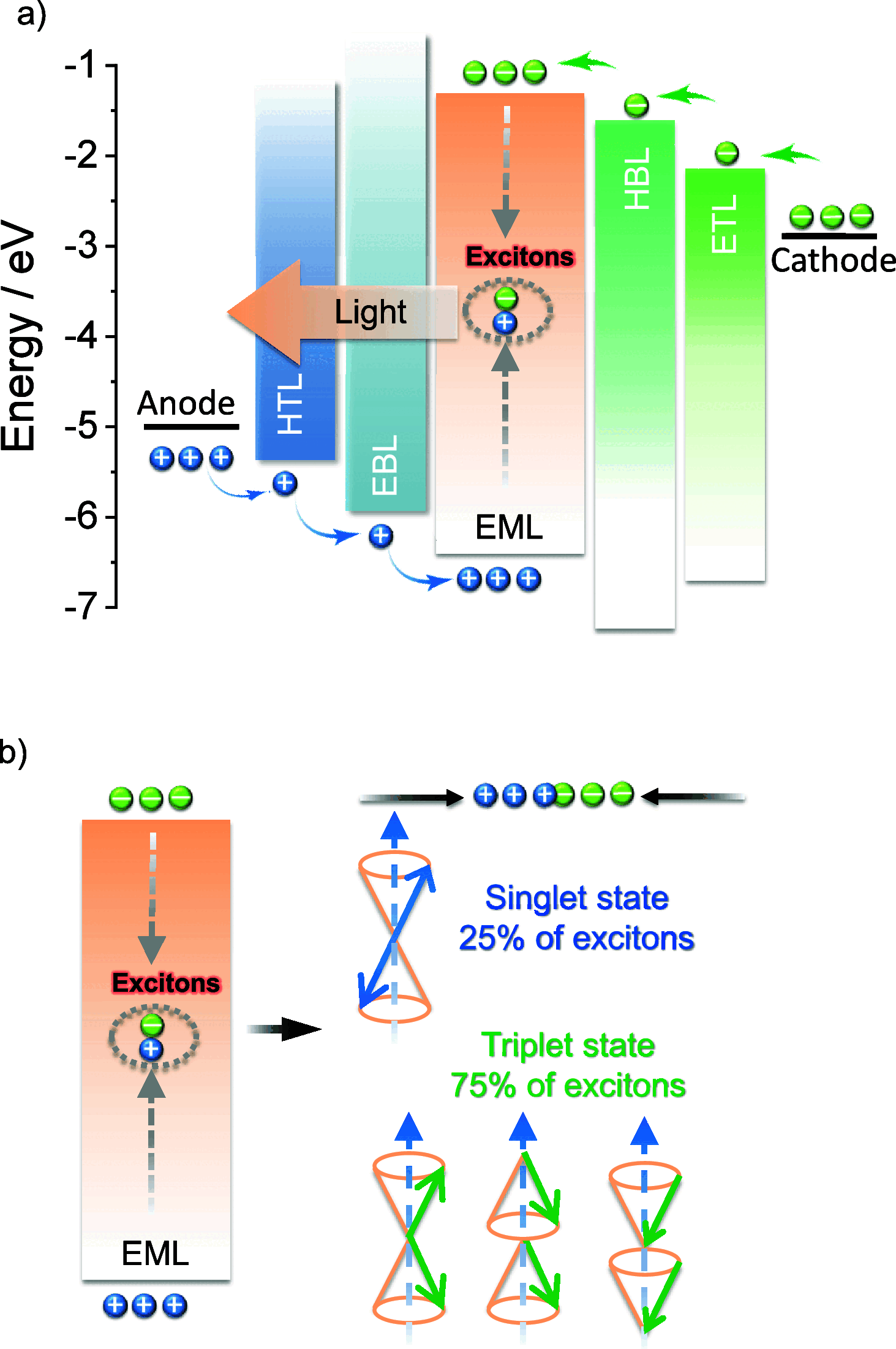

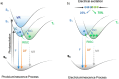

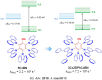
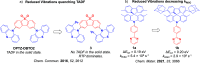
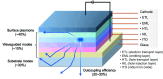




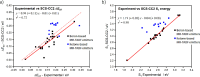
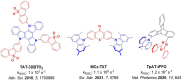

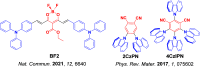
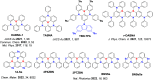
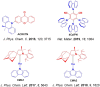
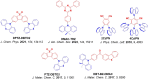


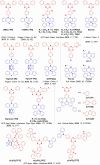
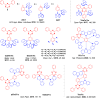
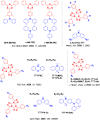
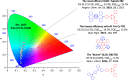
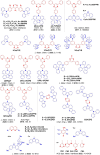
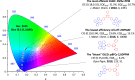
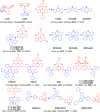
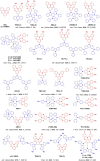
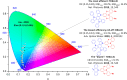
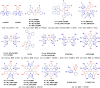
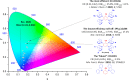



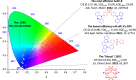

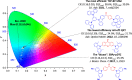

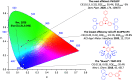

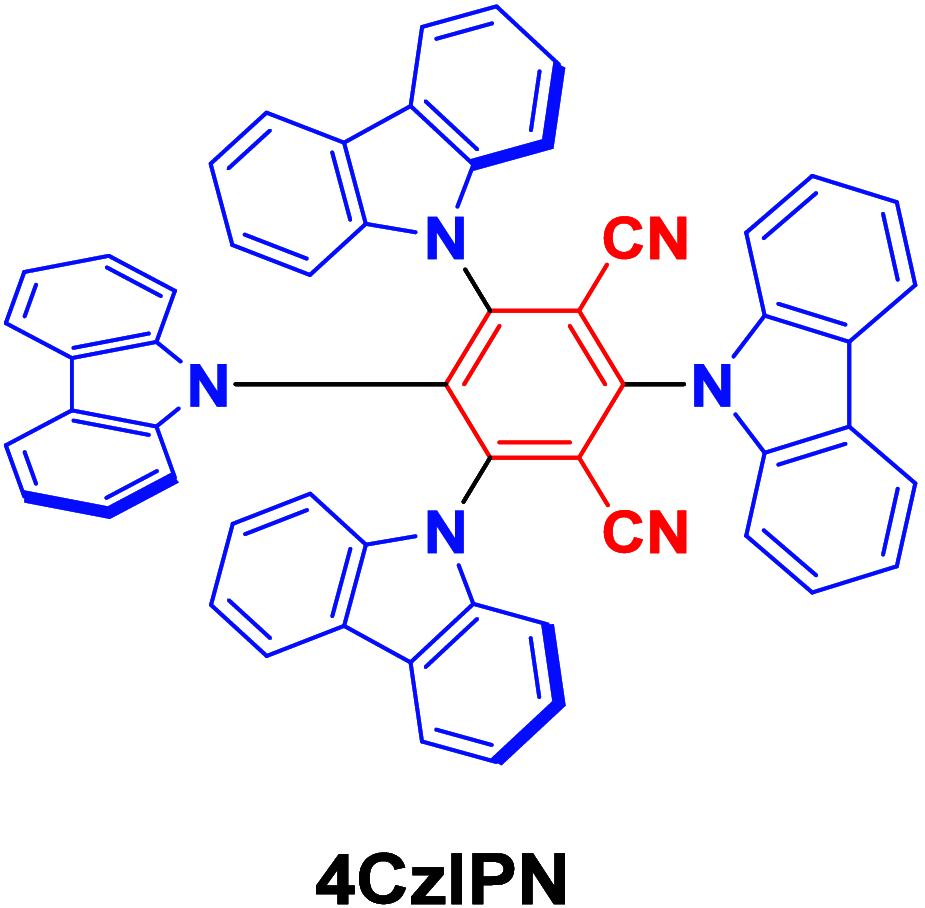
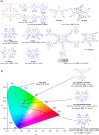




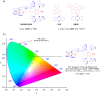
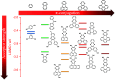
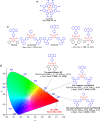
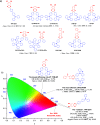


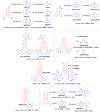
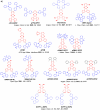
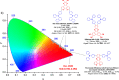
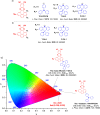
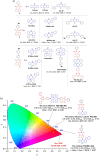
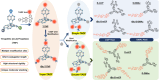
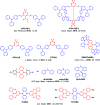
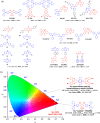

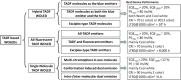
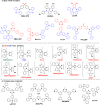
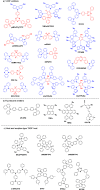


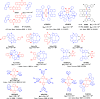
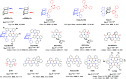
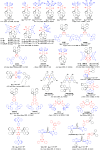

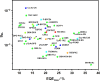
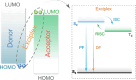

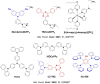
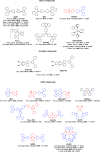


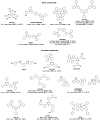
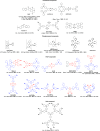
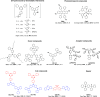
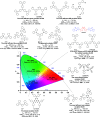

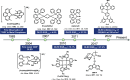
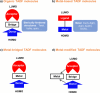





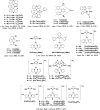
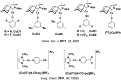

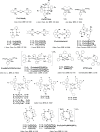
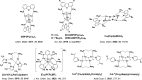
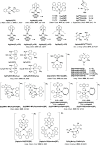

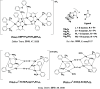

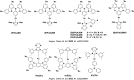

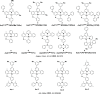
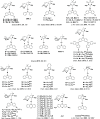



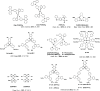

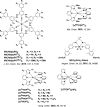

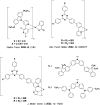


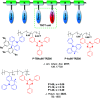


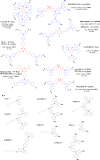
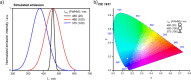


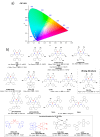
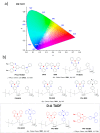
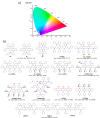


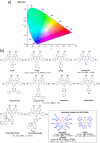
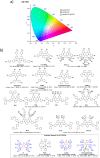
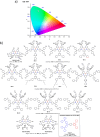
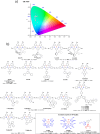
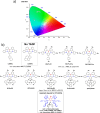

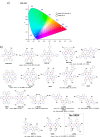

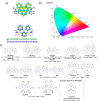
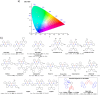
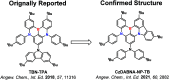
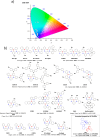
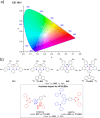

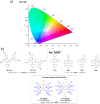

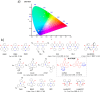
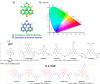


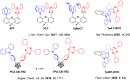


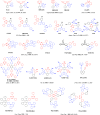

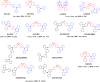

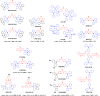


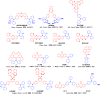
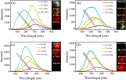


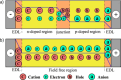
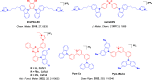
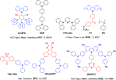
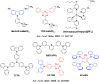
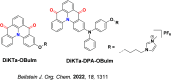
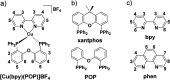




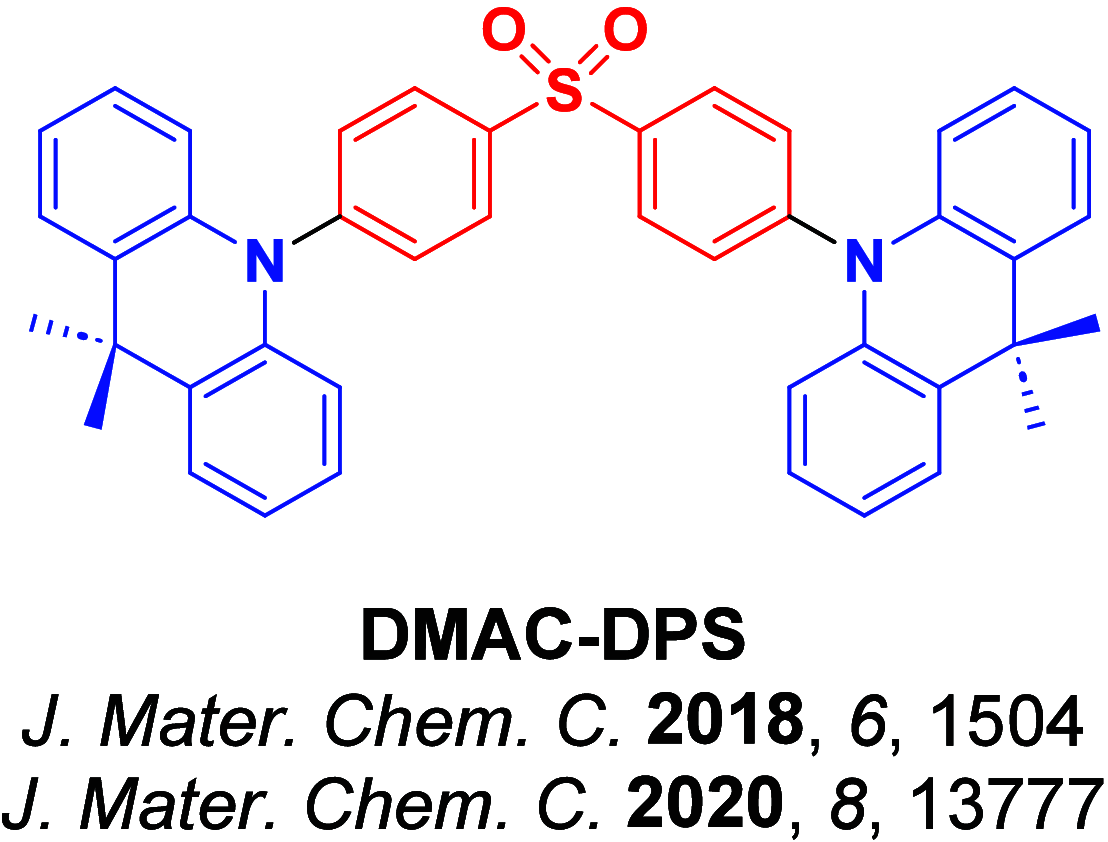
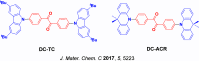
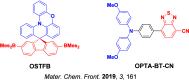
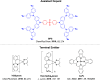
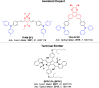
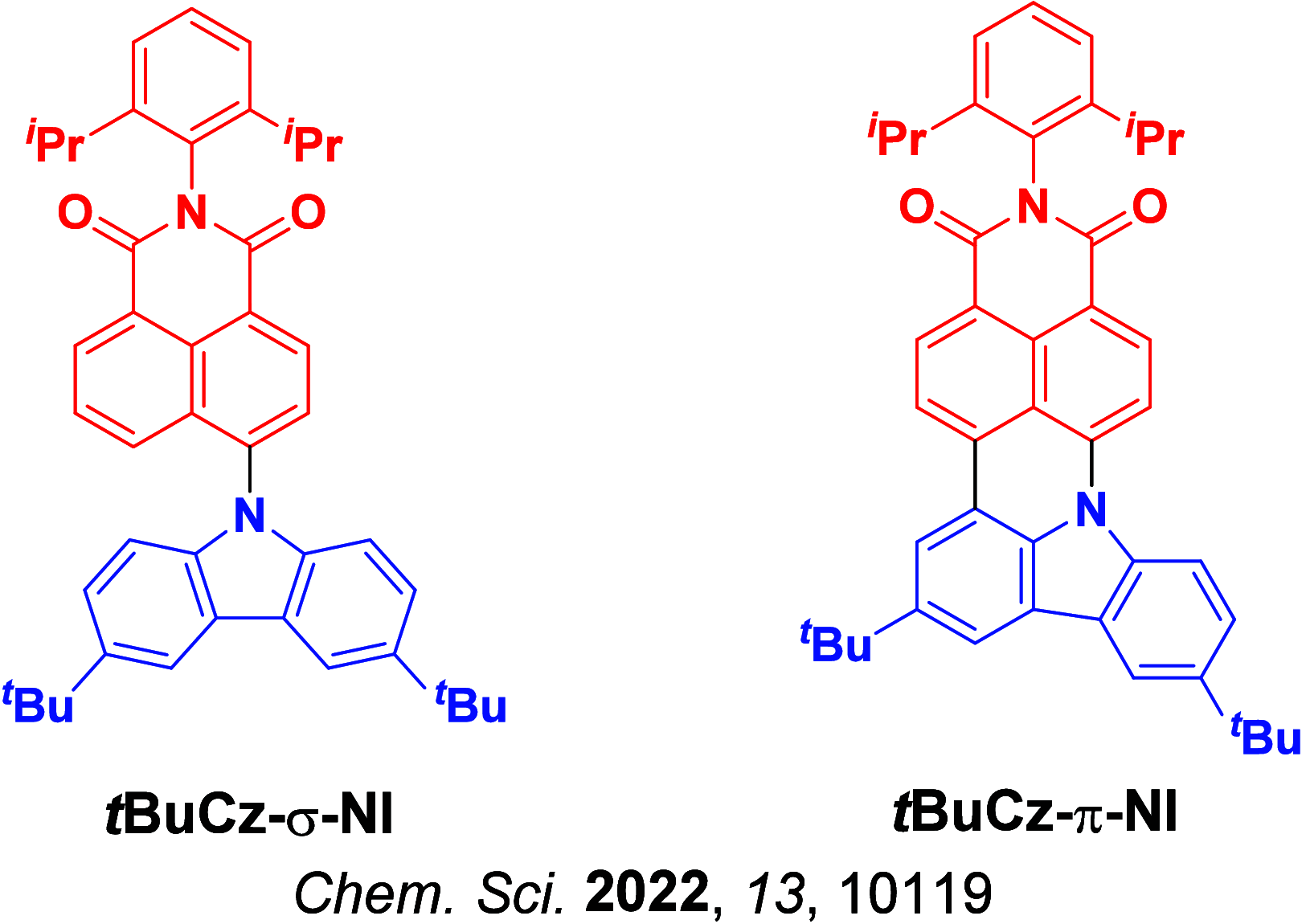
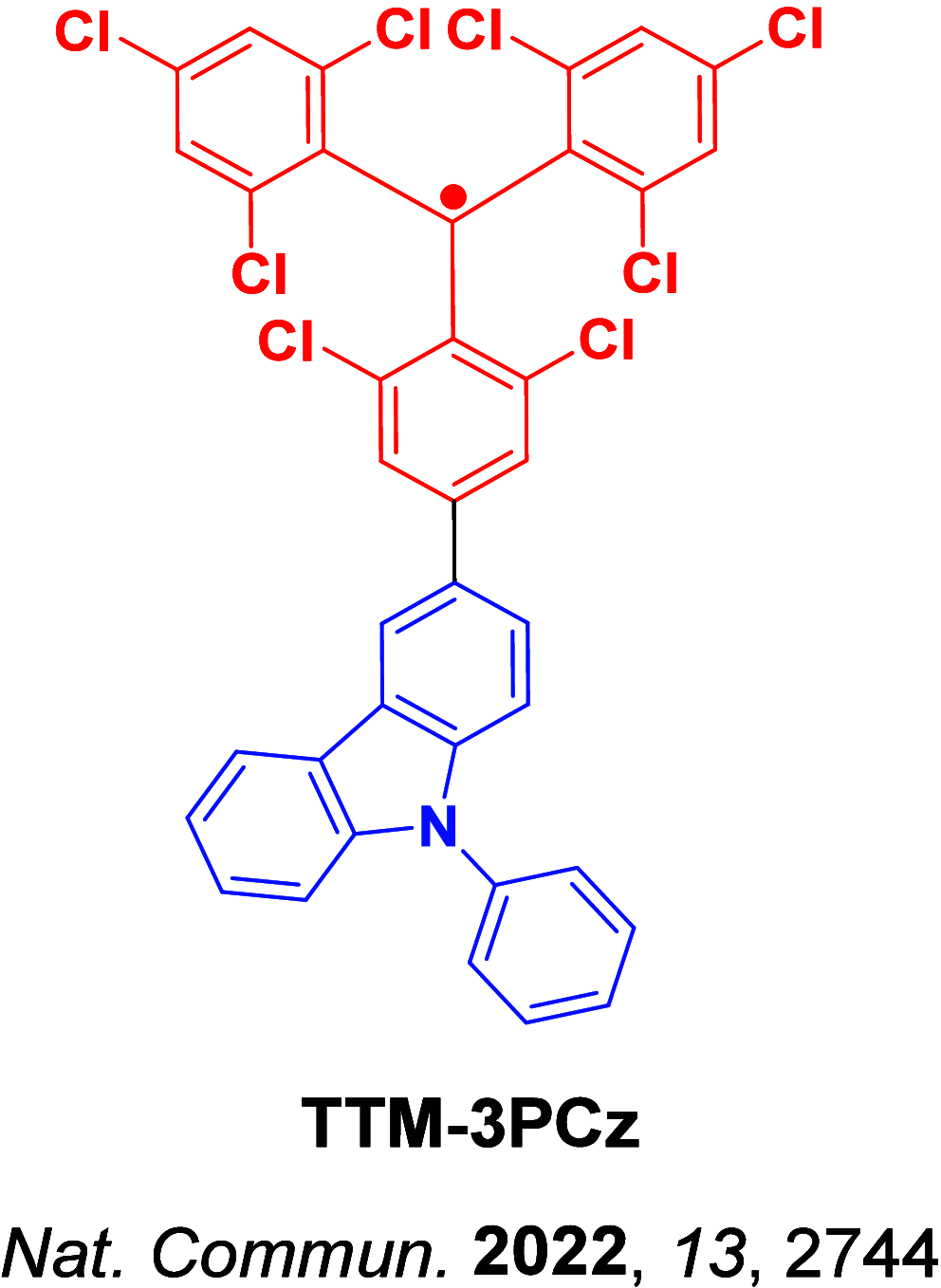

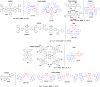
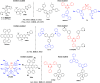
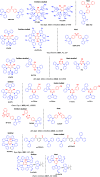

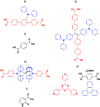




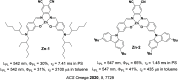
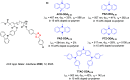

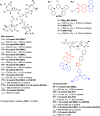
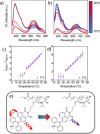



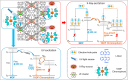

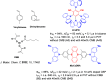
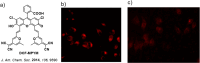
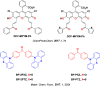




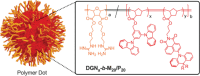
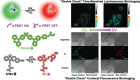
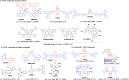
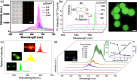
References
-
- Son, W. OLED Adoption by Smartphones to Slow Down in 2022. https://www.counterpointresearch.com/insights/oled-adoption-smartphones-... (accessed 23-01-2023).
-
- Research, G. V. Smartwatch Market Size, Share & Trends Analysis Report by Price Band, by Display Technology (LCD, OLED), by Region, and Segment Forecasts, 2022 - 2030. https://www.grandviewresearch.com/industry-analysis/smartwatches-market (accessed 20-01-2023).
-
- Mertens, R. DSCC: The OLED Market Will Grow 2% in 2022, Led by Strong Demand for IT Panels. https://www.OLED-info.com/dscc-OLED-market-will-grow-2-2022-led-strong-d... (accessed 17-02-2023).
-
- Ameco Research Automotive OLED Market Size to Reach Usd 9 Billion 2030. https://www.openpr.com/news/2934154/automotive-OLED-market-size-to-reach... (accessed 17-03-2023).
-
- Köhler A., Bässler H.. Triplet States in Organic Semiconductors. Mater. Sci. Eng., R. 2009;66:71–109. doi: 10.1016/j.mser.2009.09.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

