In vitro hydrolysis of areca nut xenobiotics in human liver
- PMID: 39667079
- PMCID: PMC11974249
- DOI: 10.1016/j.dmpk.2024.101039
In vitro hydrolysis of areca nut xenobiotics in human liver
Abstract
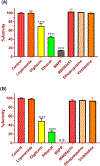
Areca nut (AN) is a substance of abuse consumed by millions worldwide, in spite of established oral and systemic toxicities associated with its use. Previous research demonstrates methyl ester alkaloids in the AN, such as arecoline and guvacoline, exhibit mood-altering and toxicological effects. Nonetheless, their metabolism has not been fully elucidated in humans. In the present study, an HPLC-UV bioanalytical method was developed to evaluate the hydrolytic kinetics and clearance rates of arecoline and guvacoline in human liver microsomes (HLM) and cytosol (HLC). The bioassay was capable of quantifying arecoline and guvacoline (and carboxylate metabolites arecaidine and guvacine, respectively) with good sensitivity, accuracy, and precision. Kinetics of arecoline and guvacoline hydrolysis best followed the Michaelis-Menten model. Apparent intrinsic clearance (Clint.in vivo) of arecoline was 57.8 ml/min/kg in HLM and 11.6 mL/min/kg in HLC, a 5-fold difference. Unexpectedly, guvacoline was dramatically less hydrolyzed than arecoline in both HLM and HLC, with Clint.in vivo estimates of 0.654 ml/min/kg and 0.466 ml/min/kg, respectively. These results demonstrate, for the first time, arecoline undergoes significant hydrolysis with high clearance rates in the liver. Furthermore, differential tissue metabolic rates and utilization of specific esterase inhibitors unequivocally demonstrated arecoline is a substrate for CES1 and not CES2.
Keywords: Areca nut; Arecoline; Betel nut; Enzyme kinetics; Esterase; Guvacoline; HPLC; Hydrolysis; Metabolism.
Copyright © 2024 The Japanese Society for the Study of Xenobiotics. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest There are no conflicts of interest to disclose.
Figures




 ); (B) Comparison of amount of arecaidine formed over 90 min in the presence of HLM (○) or HLC (
); (B) Comparison of amount of arecaidine formed over 90 min in the presence of HLM (○) or HLC ( ). Each point represents the mean (±SD) of at least triplicate experiments.
). Each point represents the mean (±SD) of at least triplicate experiments.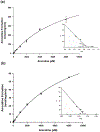

Similar articles
-
Metabolism of the areca alkaloids - toxic and psychoactive constituents of the areca (betel) nut.Drug Metab Rev. 2022 Nov;54(4):343-360. doi: 10.1080/03602532.2022.2075010. Epub 2022 May 29. Drug Metab Rev. 2022. PMID: 35543097 Review.
-
Analysis of Alkaloids in Areca Nut-Containing Products by Liquid Chromatography-Tandem Mass Spectrometry.J Agric Food Chem. 2017 Mar 8;65(9):1977-1983. doi: 10.1021/acs.jafc.6b05140. Epub 2017 Feb 23. J Agric Food Chem. 2017. PMID: 28190359 Free PMC article.
-
A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse.Chem Res Toxicol. 2006 Jun;19(6):818-27. doi: 10.1021/tx0600402. Chem Res Toxicol. 2006. PMID: 16780361 Free PMC article.
-
Comparison of the psychoactive activity of four primary Areca nut alkaloids in zebrafish by behavioral approach and molecular docking.Biomed Pharmacother. 2022 Nov;155:113809. doi: 10.1016/j.biopha.2022.113809. Epub 2022 Oct 7. Biomed Pharmacother. 2022. PMID: 36271580
-
Alkaloids and nitrosamines in betel quid: A biochemical exploration of carcinogenicity.Chem Biol Interact. 2025 Feb 1;407:111383. doi: 10.1016/j.cbi.2025.111383. Epub 2025 Jan 11. Chem Biol Interact. 2025. PMID: 39805416 Review.
References
-
- Myers AL. Metabolism of the areca alkaloids - toxic and psychoactive constituents of the areca (betel) nut. Drug Metab Rev 2022;54(4):343–60. - PubMed
-
- Peng W, Liu YJ, Wu N, Sun T, He XY, Gao YX, et al. Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol 2015;164:340–56. - PubMed
-
- Tungare S, Myers AL. Retail availability and characteristics of addictive areca nut products in a US metropolis. J Psychoactive Drugs 2021;53(3):256–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

