Prognostic value of radiologic and pathological response in colorectal cancer liver metastases upon systemic induction treatment: subgroup analysis of the CAIRO5 trial
- PMID: 39667310
- PMCID: PMC11697041
- DOI: 10.1016/j.esmoop.2024.104075
Prognostic value of radiologic and pathological response in colorectal cancer liver metastases upon systemic induction treatment: subgroup analysis of the CAIRO5 trial
Abstract
Background: RECIST may not be optimal for assessing treatment response with current systemic regimens. We evaluated RECIST, morphologic, and pathologically documented response (pathological response) in patients with initially unresectable colorectal cancer liver-only metastases (CRLM).
Patients and methods: Four hundred and eighty-nine patients from the phase III CAIRO5 trial were included who were treated with FOLFOX/FOLFIRI/FOLFOXIRI and bevacizumab or panitumumab. The association of the different response tools with overall survival (OS) was evaluated for all patients, and with early recurrence (<6 months) for patients after complete local treatment.
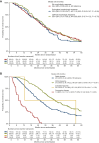
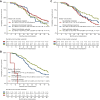
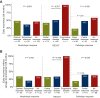
Results: In the overall population, suboptimal [hazard ratio (HR) 1.10, 95% confidence interval (CI) 0.83-1.47] and optimal (HR 0.95, 95% CI 0.74-1.22) morphologic response were not associated with OS compared with no response. RECIST partial response (HR 0.61, 95% CI 0.49-0.76) and progressive disease (HR 5.77, 95% CI 3.97-8.39) were associated with OS compared with stable disease. In 242 patients who underwent local treatment, suboptimal (HR 1.22, 95% CI 0.76-1.96) and optimal (HR 1.28, 95% CI 0.89-1.86) morphologic response were not associated with OS compared with no response. RECIST partial response was not significantly associated with OS (HR 0.73, 95% CI 0.52-1.01), whereas progressive disease was (HR 19.74, 95% CI 5.75-67.78), compared with stable disease. While major pathological response (HR 0.66, 95% CI 0.44-0.99) was associated with OS, partial pathological response (HR 0.82, 95% CI 0.57-1.19) was not, compared with no pathological response. Pathological response, but not morphologic response and RECIST, was significantly associated with early recurrence (P < 0.001) which occurred in 13/58 (22%) patients with major response, 29/61 (48%) patients with partial response, and 51/88 (58%) patients with no response.
Conclusions: Our results show that RECIST but not morphologic response was prognostic for OS. In patients eligible for local treatment, neither RECIST nor morphologic response were associated with early recurrence. Pathological response was associated with early recurrence but is only available post-operatively. Hence, novel preoperative parameters are warranted to predict early recurrence and prevent potentially futile liver surgery.
Keywords: RECIST; colorectal cancer; liver metastases; morphologic response; pathological response.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. - PubMed
-
- Nishioka Y., Shindoh J., Yoshioka R., et al. Radiological morphology of colorectal liver metastases after preoperative chemotherapy predicts tumor viability and postoperative outcomes. J Gastrointest Surg. 2015;19(9):1653–1661. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

