Randomized Multicenter Study to Evaluate the Efficacy and Safety of Fexuprazan According to the Timing of Dosing in Patients With Erosive Esophagitis
- PMID: 39667898
- PMCID: PMC11735194
- DOI: 10.5056/jnm24032
Randomized Multicenter Study to Evaluate the Efficacy and Safety of Fexuprazan According to the Timing of Dosing in Patients With Erosive Esophagitis
Abstract
Background/aims: Fexuprazan, a novel potassium-competitive acid blocker, was developed for treating acid-related disorders. Pharmacokinetic and pharmacodynamic properties of fexuprazan, unlike those of proton pump inhibitors, are independent of food effect. This study aims to evaluate differences in efficacy and safety of fexuprazan in patients with erosive esophagitis (EE) according to the timing of dosing.
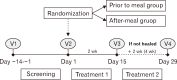
Methods: In this multicenter, open-label noninferiority study, patients who had typical reflux symptoms with endoscopically confirmed EE were randomized 1:1 to receive fexuprazan 40 mg daily 30 minutes before or after meal. Treatment was completed after 2 weeks or 4 weeks when healing was endoscopically confirmed. The primary endpoint was the proportion of patients with healed EE confirmed by endoscopy up to week 4. Safety endpoints included treatment-emergent adverse events (TEAEs).
Results: In the prior-to-meal group (n = 89) and after-meal group (n = 86), 4-week EE healing rates were 98.77% and 100.00% (difference, 0.01%; 95% CI, -0.01% to 0.04%) and 2-week EE healing rates were 95.77% and 97.14% (difference, 0.01%; 95% CI, -0.05% to 0.07%), respectively. TEAEs were 9.78% and 8.70% in the prior-to-meal group and the after-meal group, respectively.
Conclusions: Non-inferiority analysis revealed that taking fexuprazan after meal was non-inferior to taking fexuprazan before meals in patients with EE. The frequency of adverse events was similar between the 2 study groups. The drug is safe and effective for healing EE regardless of the timing of dosing.
Keywords: Esophagitis; Gastroesophageal reflux; Potassium-competitive acid blocker; Proton pumps.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources

