FrozONE: quick cell nucleus enrichment for comprehensive proteomics analysis of frozen tissues
- PMID: 39667914
- PMCID: PMC11638322
- DOI: 10.26508/lsa.202403130
FrozONE: quick cell nucleus enrichment for comprehensive proteomics analysis of frozen tissues
Abstract
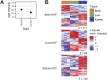
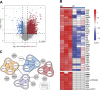
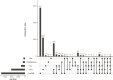
Subcellular fractionation allows for the investigation of compartmentalized processes in individual cellular organelles. Nuclear enrichment methods commonly employ the use of density gradients combined with ultracentrifugation for freshly isolated tissues. Although it is broadly used in combination with proteomics, this approach poses several challenges when it comes to scalability and applicability for frozen material. To overcome these limitations, we developed FrozONE (Frozen Organ Nucleus Enrichment), a nucleus enrichment and proteomics workflow for frozen tissues. By extensively benchmarking our workflow against alternative methods, we showed that FrozONE is a faster, simpler, and more scalable alternative to conventional ultracentrifugation methods. FrozONE allowed for the study, profiling, and classification of nuclear proteomes in different tissues with complex cellular heterogeneity, ensuring optimal nucleus enrichment from different cell types and quantitative resolution for low abundant proteins. In addition to its performance in healthy mouse tissues, FrozONE proved to be very efficient for the characterization of liver nuclear proteome alterations in a pathological condition, diet-induced nonalcoholic steatohepatitis.
© 2024 Huschet et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Appel GB, Radhakrishnan J (2012) Glomerular disorders and nephrotic syndromes. In Goldman’s Cecil Medicine, 24th edn, pp 761–771. Philadelphia, PA: Elsevier Inc.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
