Olaparib as Treatment Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer: Phase III SOLO3 Study Final Overall Survival Results
- PMID: 39668137
- PMCID: PMC12005862
- DOI: 10.1200/JCO.24.00933
Olaparib as Treatment Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer: Phase III SOLO3 Study Final Overall Survival Results
Abstract
Olaparib treatment significantly improved objective response rate (primary end point) and progression-free survival versus nonplatinum chemotherapy in patients with BRCA-mutated platinum-sensitive relapsed ovarian cancer in the open-label phase III SOLO3 trial (ClinicalTrials.gov identifier: NCT02282020). We report final overall survival (OS; prespecified secondary end point), post hoc OS analysis by number of previous chemotherapy lines, and exploratory BRCA reversion mutation analysis. Two hundred sixty-six patients were randomly assigned 2:1 to olaparib tablets (300 mg twice daily; n = 178) or physician's choice of single-agent nonplatinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan; n = 88). OS was similar with olaparib versus chemotherapy (hazard ratio [HR], 1.07 [95% CI, 0.76 to 1.49]; P = .71, median 34.9 and 32.9 months, respectively, full analysis set). OS with olaparib was favorable in patients with two previous chemotherapy lines (HR, 0.83 [olaparib v chemotherapy] [95% CI, 0.51 to 1.38]; median 37.9 v 28.8 months); however, a potential detrimental effect was seen in patients with at least three previous chemotherapy lines (HR, 1.33 [95% CI, 0.84 to 2.18]; median 29.9 v 39.4 months). BRCA reversion mutations might have contributed to this finding. No patient randomly assigned to olaparib with a BRCA reversion mutation detected at baseline (6 of 170 [3.5%]) achieved an objective tumor response.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
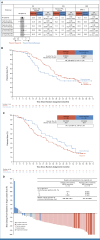
References
-
- AstraZeneca : LYNPARZA® (Olaparib) Tablets, for Oral Use: Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208558s009lbl.pdf
-
- Tobalina L, Armenia J, Irving E, et al. : A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 32:103-112, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

