Early resistance rehabilitation improves functional regeneration following segmental bone defect injury
- PMID: 39668145
- PMCID: PMC11638264
- DOI: 10.1038/s41536-024-00377-9
Early resistance rehabilitation improves functional regeneration following segmental bone defect injury
Abstract
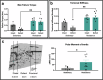
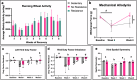
Many studies have explored different loading and rehabilitation strategies, yet rehabilitation intensity and its impact on the local strain environment and bone healing have largely not been investigated. This study combined implantable strain sensors and subject-specific finite element models in a 2 mm rodent segmental bone defect model. After injury animals were underwent high or low intensity rehabilitation. High intensity rehabilitation increased local strains within the regenerative niche by an average of 44% compared to the low intensity rehabilitation. Finite element modeling demonstrated that resistance rehabilitation significantly increased compressive strain by a factor of 2.0 at week 2 and 4.45 after 4 weeks of rehabilitation. Animals that underwent resistance running had the greatest bone volume and improved functional recovery with regenerated femurs that matched intact failure torque and torsional stiffness values. These results demonstrate the potential for early resistance rehabilitation to improve bone healing.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Robert Guldberg, Ghee Ong, and Salil Karipott have equity in Penderia Inc., a company that develops implantable orthopedic implants for clinical use. No other authors declare conflict of interests.
Figures



Update of
-
Early Resistance Rehabilitation Improves Functional Regeneration Following Segmental Bone Defect Injury.Res Sq [Preprint]. 2023 Oct 4:rs.3.rs-3236150. doi: 10.21203/rs.3.rs-3236150/v1. Res Sq. 2023. Update in: NPJ Regen Med. 2024 Dec 12;9(1):38. doi: 10.1038/s41536-024-00377-9. PMID: 37886569 Free PMC article. Updated. Preprint.
Similar articles
-
Development of a subject-specific finite element analysis workflow to assess local biomechanics during segmental bone defect healing.J Mech Behav Biomed Mater. 2025 Sep;169:107065. doi: 10.1016/j.jmbbm.2025.107065. Epub 2025 May 19. J Mech Behav Biomed Mater. 2025. PMID: 40449116
-
Early Resistance Rehabilitation Improves Functional Regeneration Following Segmental Bone Defect Injury.Res Sq [Preprint]. 2023 Oct 4:rs.3.rs-3236150. doi: 10.21203/rs.3.rs-3236150/v1. Res Sq. 2023. Update in: NPJ Regen Med. 2024 Dec 12;9(1):38. doi: 10.1038/s41536-024-00377-9. PMID: 37886569 Free PMC article. Updated. Preprint.
-
Leptin Enhances M1 Macrophage Polarization and Impairs Tendon-Bone Healing in Rotator Cuff Repair: A Rat Model.Clin Orthop Relat Res. 2025 May 1;483(5):939-951. doi: 10.1097/CORR.0000000000003428. Epub 2025 Feb 19. Clin Orthop Relat Res. 2025. PMID: 39982019
-
Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2016 Dec 8;12(12):CD005305. doi: 10.1002/14651858.CD005305.pub4. Cochrane Database Syst Rev. 2016. PMID: 27930803 Free PMC article.
-
Rehabilitation for ankle fractures in adults.Cochrane Database Syst Rev. 2024 Sep 23;9(9):CD005595. doi: 10.1002/14651858.CD005595.pub4. Cochrane Database Syst Rev. 2024. PMID: 39312389
Cited by
-
Development of a subject-specific finite element analysis workflow to assess local biomechanics during segmental bone defect healing.J Mech Behav Biomed Mater. 2025 Sep;169:107065. doi: 10.1016/j.jmbbm.2025.107065. Epub 2025 May 19. J Mech Behav Biomed Mater. 2025. PMID: 40449116
References
-
- Calori, G. M., Mazza, E., Colombo, M., Ripamonti, C. & Tagliabue, L. Treatment of long bone non-unions with polytherapy: Indications and clinical results. Injury42, 587–590 (2011). - PubMed
-
- Keating, J. F., Simpson, A. H. R. W. & Robinson, C. M. The management of fractures with bone loss. J. Bone Jt. Surg. Br.87-B, 142–150 (2005). - PubMed
-
- Hurkmans, H. L. et al. The difference between actual and prescribed weight bearing of total hip patients with a trochanteric osteotomy: long-term vertical force measurements inside and outside the hospital. Arch. Phys. Med. Rehabilitation88, 200–206 (2007). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

