MouseGoggles: an immersive virtual reality headset for mouse neuroscience and behavior
- PMID: 39668209
- PMCID: PMC11810773
- DOI: 10.1038/s41592-024-02540-y
MouseGoggles: an immersive virtual reality headset for mouse neuroscience and behavior
Abstract
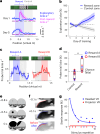
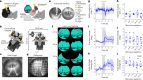
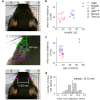
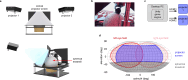
Small-animal virtual reality (VR) systems have become invaluable tools in neuroscience for studying complex behavior during head-fixed neural recording, but they lag behind commercial human VR systems in terms of miniaturization, immersivity and advanced features such as eye tracking. Here we present MouseGoggles, a miniature VR headset for head-fixed mice that delivers independent, binocular visual stimulation over a wide field of view while enabling eye tracking and pupillometry in VR. Neural recordings in the visual cortex validate the quality of image presentation, while hippocampal recordings, associative reward learning and innate fear responses to virtual looming stimuli demonstrate an immersive VR experience. Our open-source system's simplicity and compact size will enable the broader adoption of VR methods in neuroscience.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Lasztóczi, B. & Klausberger, T. Hippocampal place cells couple to three different gamma oscillations during place field traversal. Neuron91, 34–40 (2016). - PubMed
-
- Keller, G. B., Bonhoeffer, T. & Hübener, M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron74, 809–815 (2012). - PubMed
-
- Sinex, D. G., Burdette, L. J. & Pearlman, A. L. A psychophysical investigation of spatial vision in the normal and reeler mutant mouse. Vis. Res.19, 853–857 (1979). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

