How Small Proteins Adjust the Metabolism of Cyanobacteria Under Stress: The Role of Small Proteins in Cyanobacterial Stress Responses
- PMID: 39668401
- PMCID: PMC11848123
- DOI: 10.1002/bies.202400245
How Small Proteins Adjust the Metabolism of Cyanobacteria Under Stress: The Role of Small Proteins in Cyanobacterial Stress Responses
Abstract
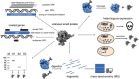
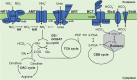
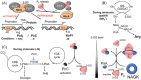
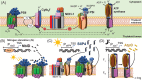
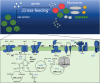
Several recently discovered small proteins of less than 100 amino acids control important, but sometimes surprising, steps in the metabolism of cyanobacteria. There is mounting evidence that a large number of small protein genes have also been overlooked in the genome annotation of many other microorganisms. Although too short for enzymatic activity, their functional characterization has frequently revealed the involvement in processes such as signaling and sensing, interspecies communication, stress responses, metabolism, regulation of transcription and translation, and in the formation of multisubunit protein complexes. Cyanobacteria are the only prokaryotes that perform oxygenic photosynthesis. They thrive under a wide variety of conditions as long as there is light and must cope with dynamic changes in the environment. To acclimate to these fluctuations, frequently small regulatory proteins become expressed that target key enzymes and metabolic processes. The consequences of their actions are profound and can even impact the surrounding microbiome. This review highlights the diverse functions of recently discovered small proteins that control cyanobacterial metabolism. It also addresses why many of these proteins have been overlooked so far and explores the potential for implementing metabolic engineering strategies to improve the use of cyanobacteria in biotechnological applications.
Keywords: biotechnology; cyanobacteria; energy metabolism; metabolic regulation; photosynthesis; small proteins; stress acclimation.
© 2024 The Author(s). BioEssays published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Houmard J. (1995). “How Do Cyanobacteria Perceive and Adjust to Their Environment?” in Molecular Ecology of Aquatic Microbes, ed. Joint I. (Berlin, Heidelberg: Springer, 1995): 153–170, 10.1007/978-3-642-79923-5_9. - DOI
-
- Gaysina L. A., Saraf A., and Singh P., “Cyanobacteria in Diverse Habitats,” in Cyanobacteria (Cambridge, MA, US: Academic Press/Elsevier, 2019): 1–28, 10.1016/B978-0-12-814667-5.00001-5. - DOI
-
- Ward D. M., Castenholz R. W., and Miller S. R. (2012). “Cyanobacteria in Geothermal Habitats,” in Ecology of Cyanobacteria II, ed. Whitton B. A. (Dordrecht, Netherlands: Springer, 2012): 39–63, 10.1007/978-94-007-3855-3_3. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

