Fine-tuning of Fgf8 morphogen gradient by heparan sulfate proteoglycans in the extracellular matrix
- PMID: 39668564
- PMCID: PMC11947464
- DOI: 10.1016/j.bpj.2024.12.009
Fine-tuning of Fgf8 morphogen gradient by heparan sulfate proteoglycans in the extracellular matrix
Abstract
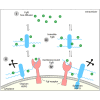
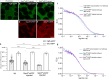
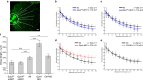
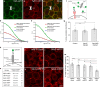
Embryonic development is orchestrated by the action of morphogens, which spread out from a local source and activate, in a field of target cells, different cellular programs based on their concentration gradient. Fibroblast growth factor 8 (Fgf8) is a morphogen with important functions in embryonic organizing centers. It forms a gradient in the extracellular space by free diffusion, interaction with the extracellular matrix (ECM), and receptor-mediated endocytosis. However, morphogen gradient regulation by ECM is still poorly understood. Here, we show that specific heparan sulfate proteoglycans (HSPGs) bind Fgf8 with different affinities directly in the ECM of living zebrafish embryos, thus affecting its diffusion and signaling. Using single-molecule fluorescence correlation spectroscopy, we quantify this binding in vivo, and find two different modes of interaction. First, reducing or increasing the concentration of specific HSPGs in the extracellular space alters Fgf8 diffusion and, thus, its gradient shape. Second, ternary complex formation of Fgf8 ligand with Fgf receptors and HSPGs at the cell surface requires HSPG attachment to the cell membrane. Together, our results show that graded Fgf8 morphogen distribution is achieved by constraining free Fgf8 diffusion through successive interactions with HSPGs at the cell surface and in ECM space.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The author declares no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

