Electrophysiological Monitoring of Asymptomatic Transthyretin Mutation Carriers
- PMID: 39668650
- PMCID: PMC11708451
- DOI: 10.1002/mus.28318
Electrophysiological Monitoring of Asymptomatic Transthyretin Mutation Carriers
Abstract
Introduction/aims: It is imperative to screen asymptomatic carriers of transthyretin (TTR) mutations to initiate treatment early. The protocol for repeated electrodiagnostic (EDX) assessments over time lacks standardization. Our aim was to report the electrophysiological evolution of a cohort of asymptomatic carriers and to determine which biomarkers were most sensitive to change.
Methods: We performed a retrospective review of medical records of asymptomatic carriers identified by screening families with amyloid neuropathy. Carriers who underwent two EDX assessments with a minimum 1-year interval between studies were selected. EDX included analysis of median, ulnar, tibial, fibular and sural nerves, motor unit number index (MUNIX), electrochemical skin conductance, sympathetic skin response, and heart rate variability on deep breathing. Measurements were compared at first and second examinations.
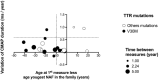
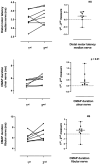
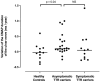
Results: Twenty-three carriers were included with a median age of 49 years (interquartile range 37-58). Median time between examinations was 3 years (2-4). Compound muscle and sensory nerve action potential (CMAP and SNAP) amplitudes, nerve conduction velocities, autonomic small fiber testing and MUNIX remained stable except for motor distal latency of the median nerve (+0.07 ms/year) and CMAP duration of the ulnar (+0.10 ms/year) and fibular (+0.12 ms/year) nerves. The CMAP duration of the ulnar nerve was the most sensitive biomarker to change when performed within 10 years preceding the age of the youngest case in the family, with a standardized response mean of 0.91.
Discussion: Nerve conduction parameters remain relatively stable in asymptomatic TTR carriers. Changes can only be detected using multimodal and extensive electrophysiological tests.
Keywords: asymptomatic carriers; electrophysiological monitoring; familial amyloid polyneuropathy; small fiber testing; transthyretin.
© 2024 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Coelho T., Conceição I., Waddington‐Cruz M., et al., “A Natural History Analysis of Asymptomatic TTR Gene Carriers as They Develop Symptomatic Transthyretin Amyloidosis in the Transthyretin Amyloidosis Outcomes Survey (THAOS),” Amyloid 29, no. 4 (2022): 228–236. - PubMed
-
- Conceição I., Damy T., Romero M., et al., “Early Diagnosis of ATTR Amyloidosis Through Targeted Follow‐Up of Identified Carriers of TTR Gene Mutations*,” Amyloid 26, no. 1 (2019): 3–9. - PubMed
-
- Adams D., Tournev I. L., Taylor M. S., et al., “Efficacy and Safety of Vutrisiran for Patients With Hereditary Transthyretin‐Mediated Amyloidosis With Polyneuropathy: A Randomized Clinical Trial,” Amyloid 30, no. 1 (2023): 18–26. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

