Association Between Serum Ustekinumab Concentrations and Endoscopic Disease Activity in Moderate-to-Severe Crohn's Disease Patients
- PMID: 39668980
- PMCID: PMC11635169
- DOI: 10.1093/crocol/otae071
Association Between Serum Ustekinumab Concentrations and Endoscopic Disease Activity in Moderate-to-Severe Crohn's Disease Patients
Abstract
Background/aims: The role of ustekinumab therapeutic drug monitoring in patients with Crohn's disease (CD) remains ambiguous. Examination of the association serum ustekinumab concentrations and endoscopic outcomes has yielded inconsistent results. Our study examined whether serum ustekinumab concentrations were associated with endoscopic healing in patients with moderate-to-severe CD.
Methods: This was a cross-sectional study of adult patients with CD on maintenance ustekinumab. Patients were included if they had serum ustekinumab concentrations and endoscopic evaluation taken within 4 months of each other. Endoscopic healing was defined as absence of ulceration on endoscopy or Simplified Endoscopic Score for Crohn's disease (SES-CD) < 3. Quartile analysis of drug levels was performed, and receiver operating characteristic curve was calculated. Multivariate logistic regression assessed for the probability of endoscopic healing based on serum ustekinumab concentration.
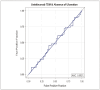
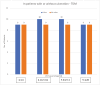
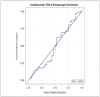
Results: Seventy-four patients were included in the final analysis. The mean serum ustekinumab concentration of the population was 6.10 mcg/mL. Serum ustekinumab concentration did not predict endoscopic remission based on either the absence of ulceration or SES-CD < 3. There was no difference in the frequency of ulceration at increasing serum ustekinumab concentrations. There was no threshold serum ustekinumab concentration associated with the absence of ulceration (area under the curve [AUC] = 0.50) or SES-CD < 3 (AUC = 0.49).
Conclusions: Our study found no association between serum ustekinumab concentrations and endoscopic remission in patients with CD. Exploration of mechanisms accounting for this lack of association is warranted.
Keywords: Crohn’s disease; biologic; endoscopy.
© The Author(s) 2024. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
D.M.P.D.F., B.A., R.Y., Y.A., P.G., A.B., and G.W. have no COI to declare. W.A. served as speaker, advisory board member, and/or clinical investigator for Abbvie, Amgen, BMS, Dynacare, Eli-Lilly, Janssen, Merck, Novartis, Pfizer, Prometheus, Sandoz, Sanofi, and Takeda. P.L.L. has been a speaker and/or advisory board member: AbbVie, Amgen, BioJamp, Bristol Myers Squibb, Fresenius Kabi, Genetech, Gilead, Janssen, Merck, Mylan, Organon, Pendopharm, Pfizer, Roche, Sandoz, Takeda, Tillots, and Viatris, and has received unrestricted research grant: AbbVie, Gilead, Takeda, and Pfizer. T.B. acted as a speaker or advisor for Abbvie, Alimentiv, Amgen, Bristol-Myers-Squibb, Eli Lilly, Ferring, Fresenius Kabi, Gilead, Iterative Scopes, Janssen, Pendopharm, Merck, Pentax, Pfizer, Roche, Sandoz, Takeda, and Viatris.
Figures

References
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L.. Crohn’s disease. Lancet. 2017;389(10080):1741-1755. doi: https://doi.org/ 10.1016/S0140-6736(16)31711-1 - DOI - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi: https://doi.org/ 10.1053/j.gastro.2020.12.031. - DOI - PubMed
-
- Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15(9):1295-1301. doi: https://doi.org/ 10.1002/ibd.20927 - DOI - PubMed
-
- Shah SC, Colombel JF, Sands BE, Narula N.. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43(3):317-333. doi: https://doi.org/ 10.1111/apt.13475 - DOI - PubMed
-
- Baert F, Moortgat L, Van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138(2):463-8; quiz e10. doi: https://doi.org/ 10.1053/j.gastro.2009.09.056 - DOI - PubMed
LinkOut - more resources
Full Text Sources
