C9orf72 repeat expansion creates the unstable folate-sensitive fragile site FRA9A
- PMID: 39669124
- PMCID: PMC11632612
- DOI: 10.1093/narmme/ugae019
C9orf72 repeat expansion creates the unstable folate-sensitive fragile site FRA9A
Abstract
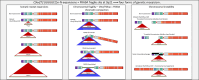
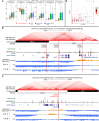
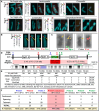
The hyper-unstable Chr9p21 locus, harbouring the interferon gene cluster, oncogenes and C9orf72, is linked to multiple diseases. C9orf72 (GGGGCC)n expansions (C9orf72Exp) are associated with incompletely penetrant amyotrophic lateral sclerosis, frontotemporal dementia and autoimmune disorders. C9orf72Exp patients display hyperactive cGAS-STING-linked interferon immune and DNA damage responses, but the source of immunostimulatory or damaged DNA is unknown. Here, we show C9orf72Exp in pre-symptomatic and amyotrophic lateral sclerosis-frontotemporal dementia patient cells and brains cause the folate-sensitive chromosomal fragile site, FRA9A. FRA9A centers on >33 kb of C9orf72 as highly compacted chromatin embedded in an 8.2 Mb fragility zone spanning 9p21, encompassing 46 genes, making FRA9A one of the largest fragile sites. C9orf72Exp cells show chromosomal instability, heightened global- and Chr9p-enriched sister-chromatid exchanges, truncated-Chr9s, acentric-Chr9s and Chr9-containing micronuclei, providing endogenous sources of damaged and immunostimulatory DNA. Cells from one C9orf72Exp patient contained a highly rearranged FRA9A-expressing Chr9 with Chr9-wide dysregulated gene expression. Somatic C9orf72Exp repeat instability and chromosomal fragility are sensitive to folate deficiency. Age-dependent repeat instability, chromosomal fragility and chromosomal instability can be transferred to CNS and peripheral tissues of transgenic C9orf72Exp mice, implicating C9orf72Exp as the source. Our results highlight unappreciated effects of C9orf72 expansions that trigger vitamin-sensitive chromosome fragility, adding structural variations to the disease-enriched 9p21 locus, and likely elsewhere.
© The Author(s) 2024. Published by Oxford University Press on behalf of NAR Molecular Medicine.
Figures





Update of
-
C9orf72 expansion creates the unstable folate-sensitive fragile site FRA9A.bioRxiv [Preprint]. 2024 Oct 29:2024.10.26.620312. doi: 10.1101/2024.10.26.620312. bioRxiv. 2024. Update in: NAR Mol Med. 2024 Nov 12;1(4):ugae019. doi: 10.1093/narmme/ugae019. PMID: 39569145 Free PMC article. Updated. Preprint.
References
-
- Fredi M., Cavazzana I., Biasiotto G., Filosto M., Padovani A., Monti E., Tincani A., Franceschini F., Zanella I. C9orf72 intermediate alleles in patients with amyotrophic lateral sclerosis, systemic lupus erythematosus, and rheumatoid arthritis. Neuromol. Med. 2019; 21:150–159. - PubMed
-
- Miller Z.A., Sturm V.E., Camsari G.B., Karydas A., Yokoyama J.S., Grinberg L.T., Boxer A.L., Rosen H.J., Rankin K.P., Gorno-Tempini M.L. et al. Increased prevalence of autoimmune disease within C9 and FTD/MND cohorts: completing the picture. Neurol. Neuroimmunol. Neuroinflamm. 2016; 3:e301. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
