The diagnosis communication process in spinal muscular atrophy: A cross-cutting view of the new challenges facing the therapeutic era
- PMID: 39669251
- PMCID: PMC11613608
- DOI: 10.1016/j.gimo.2023.100825
The diagnosis communication process in spinal muscular atrophy: A cross-cutting view of the new challenges facing the therapeutic era
Abstract
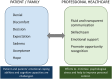
Spinal muscular atrophy (SMA) is a prevalent severe genetic condition that follows an autosomal recessive inheritance pattern. Over the last decade, advances in innovative therapies have improved the course of the disease for many patients. There is evidence that early diagnosis and therapeutic intervention contribute toward better outcomes for these patients. The implementation of SMA newborn screening allows presymptomatic diagnosis leading to new communication scenarios, which poses opportunities and challenges when discussing possible treatment and evolution with families. Communication skills are essential to transmit accurate and comprehensive information to promote better coping and facilitate shared treatment decisions considering patient, family, and physicians' points of view. The role of professionals is increasing as patients live longer and present evolving phenotypes. Therefore, multidisciplinary follow-up has emerged as an essential component of the standard of care protocol for patients with SMA. On the other hand, issues regarding communication of the diagnosis to a new patient still deserve a thorough discussion to better accommodate the complexity of the different situations. We present this review as a cross-cutting perspective involving health care practitioners, genetic counselors, psychologists, and caregivers to further elaborate and guide the communication process of an SMA diagnosis under several settings.
Keywords: Communication of diagnosis; Genetic counseling; Psychological impact; Spinal muscular atrophy; Treatment decision making.
© 2023 The Authors.
Conflict of interest statement
The manuscript has been seen and approved by all the authors and they have taken care to ensure the integrity of the work. Eulàlia Rovira-Moreno, Anna Abulí, Patricia Muñoz-Cabello, Marta Codina-Sola, Eva Baillès and Mencía de Lemus have no conflicts of interest or financial disclosures to report. Basil T. Darras has served as an ad hoc scientific board member for Biogen, Novartis Gene Therapies (AveXis), and Roche and Genentech, the pharmaceutical companies which manufacture nusinersen, onasemnogene abeparvovec-xioi, and risdiplam. Dr. Darras is also the FIREFISH study Steering Committee Chair for Roche, which manufactures risdiplam. Eduardo F. Tizzano has served as an ad hoc scientific board member for Biogen, Novartis Gene Therapies (AveXis), and Roche, the pharmaceutical companies which manufacture nusinersen, onasemnogene abeparvovec-xioi, and risdiplam, respectively.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

