Mucosal vaccination with outer membrane vesicles derived from Bordetella pertussis reduces nasal bacterial colonization after experimental infection
- PMID: 39669568
- PMCID: PMC11635837
- DOI: 10.3389/fimmu.2024.1506638
Mucosal vaccination with outer membrane vesicles derived from Bordetella pertussis reduces nasal bacterial colonization after experimental infection
Abstract
Introduction: We previously identified Bordetella pertussis-derived outer membrane vesicles (OMVs) as a promising immunogen for improving pertussis vaccines. In this study, we evaluated the efficacy of our vaccine prototype in immunization strategies aimed at reducing disease transmission by targeting colonization in the upper airways while maintaining protection against severe disease by reducing colonization in the lower respiratory tract.
Methods: We assessed different mucosal administration strategies in a murine model, including homologous mucosal 2-dose prime-boost schedules and heterologous prime-boost strategies combining intramuscular (IM) systemic immunization with mucosal routes (intranasal, IN; or sublingual, SL). We utilized alum and c-di-AMP as adjuvants for the systemic and mucosal formulations of the OMV vaccine prototype, respectively. A homologous prime/boost IM immunization schedule and commercial vaccines were used for comparisons.
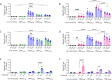
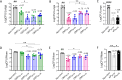
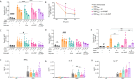
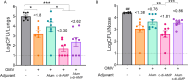
Results: All tested heterologous schemes induced higher levels of specific IgG with significant avidity, as well as higher levels of IgG1 and IgG2c, compared to the corresponding homologous prime-boost 2-dose schemes via mucosal routes (OMVIN-IN or OMVSL-SL). High IgA levels were observed post-B. pertussis challenge following OMVIN-IN treatments and heterologous treatments where the second dose was administered via a mucosal route (prime-pull scheme). Furthermore, schemes involving the intranasal route, whether in a homologous or heterologous scheme, induced the highest levels of IL-17 and IFN-γ. Accordingly, these schemes showed superior efficacy against nasal colonization than the commercial vaccines. Homologous intranasal immunization exhibited the highest protective capacity against nasal colonization while maintaining an excellent level of protection in the lower respiratory tract. To further enhance protection against nasal colonization, we performed a comparative analysis of formulations containing either single or combined adjuvants, administered via homologous intranasal route. These assays revealed that the use of alum combined with c-di-AMP, did not enhance the immune protective capacity in comparison with that observed for the formulation containing c-di-AMP alone.
Conclusions: All the experiments presented here demonstrate that the use of OMVs, regardless of the scheme applied (except for OMVSL-SL), significantly outperformed acellular pertussis (aP) vaccines, achieving a higher reduction in bacterial colonization in the upper respiratory tract (p<0.01).
Keywords: Bordetella pertussis; IgA; intranasal; mucosal; outer-membrane vesicles.
Copyright © 2024 Rudi, Gaillard, Bottero, Ebensen, Guzman and Hozbor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

