Dynamics of antibody engagement of red blood cells in vivo and in vitro
- PMID: 39669570
- PMCID: PMC11634868
- DOI: 10.3389/fimmu.2024.1475470
Dynamics of antibody engagement of red blood cells in vivo and in vitro
Abstract
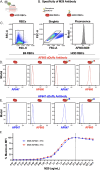
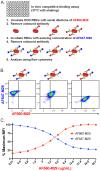
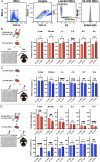
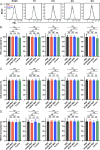
Exposure to allogenic red blood cells (RBCs), either through pregnancy or transfusion, can result in alloimmunization, which can lead to severe hemolytic transfusion reactions and pregnancy complications. Passively administered antibodies can be used to prevent alloimmunization, where steric hindrance of allogeneic epitopes has been postulated as one mechanism whereby antibody engagement may prevent RBC alloimmunization. However, the dynamics of antibody engagement on the RBC surface has remained difficult to study. To examine this, we leveraged the HOD (HEL, OVA and Duffy) model system and Fcγ receptor knockout recipients to define the dynamics of antibody engagement of the Duffy antigen in the absence of RBC clearance or antigen modulation. Using this approach, the on-rate of antibody engagement of HOD RBCs was very similar in vivo and in vitro, with high levels of antibody binding observed within minutes of HOD RBC exposure. In contrast, the off-rate of HOD RBC bound antibody was relatively slow, with appreciable dissociation not being observed for an hour. However, the dynamics of antibody interactions with HOD changed significantly when antibody decorated HOD RBCs were exposed to free antibody. Despite the presence of prebound antibody, free antibody rapidly associated with HOD RBCs, with the rate of free antibody association observed being faster in vivo than in vitro. Importantly, antibody association and dissociation occurred in the absence of any appreciable changes in RBC clearance, antigen modulation or complement deposition, suggesting that differences in antibody levels observed reflected actual differences in the dynamics of antibody binding. These results suggest that while antibodies appear to be relatively static on the cell surface once bound, antibody engagement can be quite dynamic, especially in the face of free antibody in solution. These results not only have implications in the mechanisms of antibody-mediated immunosuppression, but also the potential use of other antibody-based approaches designed to prevent hemolytic transfusion reactions or target antigens in vivo in general.
Keywords: AMIS; alloimmunization; antibody; antigen; red blood cell.
Copyright © 2024 Jajosky, Ayona, Mener, Stowell and Arthur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CD47 regulates antigen modulation and red blood cell clearance following an incompatible transfusion.Front Immunol. 2025 Apr 4;16:1548548. doi: 10.3389/fimmu.2025.1548548. eCollection 2025. Front Immunol. 2025. PMID: 40255405 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Transfusion thresholds for guiding red blood cell transfusion.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5. Cochrane Database Syst Rev. 2021. PMID: 34932836 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
Cited by
-
CD47 regulates antigen modulation and red blood cell clearance following an incompatible transfusion.Front Immunol. 2025 Apr 4;16:1548548. doi: 10.3389/fimmu.2025.1548548. eCollection 2025. Front Immunol. 2025. PMID: 40255405 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

