Isocyanate-based multicomponent reactions
- PMID: 39670166
- PMCID: PMC11635408
- DOI: 10.1039/d4ra04152f
Isocyanate-based multicomponent reactions
Abstract
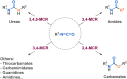
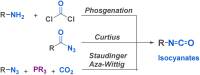
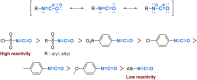
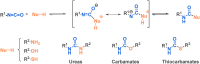
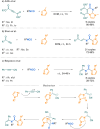
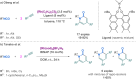
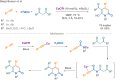
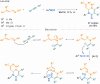
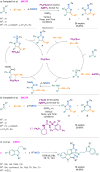
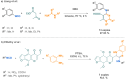
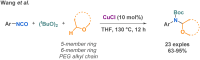
Since their discovery, multicomponent reactions have attracted significant attention due to their versatility and efficiency. This review aims to explore the latest advancements in isocyanate-based multicomponent reactions and the sophisticated chemical opportunities they present for generating molecules of interest. The added value of the methodologies described, supported by mechanism schemes, as well as scopes of application, will be discussed. These developments will be organised as the main accessible chemical functions and sorted according to their type of MCR (3, 4 or 5-MCR).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





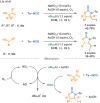

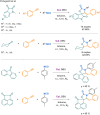
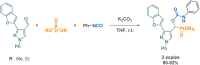
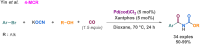
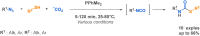
References
-
- Strecker A. Ann. Chem. Pharm. 1850;75:27–45.
-
- Strecker A. Ann. Chem. Pharm. 1854;91:349–351.
-
- Dömling A. and AlQahtani A. D., in Multicomponent Reactions in Organic Synthesis, Wiley, 1st edn, 2014, pp. 1–12
-
- Anastas P. T. and Warner J. C., Green chemistry: theory and practice, 2000
-
- Hantzsch A. Ann. Chem. Pharm. 1882;215:1–82.
Publication types
LinkOut - more resources
Full Text Sources

