Preclinical Evaluation of Bavdegalutamide (ARV-110), a Novel PROteolysis TArgeting Chimera Androgen Receptor Degrader
- PMID: 39670468
- PMCID: PMC11962395
- DOI: 10.1158/1535-7163.MCT-23-0655
Preclinical Evaluation of Bavdegalutamide (ARV-110), a Novel PROteolysis TArgeting Chimera Androgen Receptor Degrader
Abstract
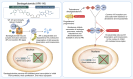
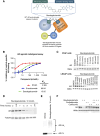
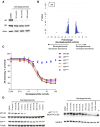
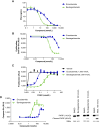
Androgen receptor (AR) signaling is the principal driver of prostate cancer, and drugs that target this pathway (e.g., abiraterone and enzalutamide) are standard treatments for metastatic hormone-sensitive prostate cancer and metastatic castration-resistant prostate cancer. However, continual evolution during prostate cancer progression can result in AR alterations (e.g., mutation, amplification, and splicing) that can cause tumors to become resistant to these therapies. Bavdegalutamide (ARV-110) is a PROteolysis TArgeting Chimera (PROTAC) protein degrader that recruits the cereblon-containing E3 ubiquitin ligase to direct the polyubiquitination and subsequent proteasomal degradation of AR. Bavdegalutamide selectively degrades wild-type AR and most clinically relevant mutants with low nanomolar potency. The advantages of the degradation mechanism of action are demonstrated by the higher activity of bavdegalutamide relative to the AR antagonist enzalutamide in cell-based systems that assess effects on PSA synthesis, proliferation of prostate cancer cells, and induction of apoptosis. In an AR-expressing patient-derived xenograft mouse model, bavdegalutamide showed substantial AR degradation and greater tumor growth inhibition compared with enzalutamide. Bavdegalutamide also showed robust tumor growth inhibition in enzalutamide- and abiraterone-resistant prostate cancer animal models and enhanced activity in combination with abiraterone. These promising preclinical data supported the clinical development of bavdegalutamide as a potential treatment for patients with prostate cancer. Bavdegalutamide was the first PROTAC protein degrader to enter human clinical trials, specifically in patients with metastatic castration-resistant prostate cancer in a phase I/II study (NCT03888612).
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
L.B. Snyder reports a patent for US10584101 issued, a patent for US10844021 issued, a patent for US11236051 issued, a patent for US11952347 issued, a patent for PCTUS2020056684 pending, a patent for PCTUS2319496 pending, and a patent for PCTUS2024025572 pending, as well as employment with Arvinas at the time of this work and stock ownership in Arvinas. R.R. Willard reports employment with Arvinas at the time of this work and stock ownership in Arvinas. D.A. Gordon reports employment with Arvinas and stock ownership in Arvinas. J. Pizzano reports employment with Arvinas and stock ownership in Arvinas at the time of this work. N. Vitale reports employment with Arvinas at the time of this work and stock ownership in Arvinas. K. Robling reports employment with Arvinas and stock ownership in Arvinas. M.A. Dorso reports employment with Arvinas and stock ownership in Arvinas. W. Moghrabi reports ownership of few stocks in Arvinas. S. Landrette reports employment with Arvinas and stock ownership in Arvinas. R. Gedrich reports a patent for PCTUS24028421 pending; employment with Arvinas and stock ownership in Arvinas at the time of this work; and current employment with PIC Therapeutics and stock ownership in PIC Therapeutics. S.H. Lee reports a patent for PCTUS2319496 pending; employment with Arvinas at the time of this work; and current employment with a biotech/pharma company other than Arvinas and stock ownership in other biotech/pharma companies. I.C.A. Taylor reports grants from department of health & human services/NIH/NCI/small business innovation research during the conduct of the study, as well as employment with Arvinas and stock ownership in Arvinas. J.G. Houston reports employment with Arvinas and stock ownership in Arvinas. T. K. Neklesa reports employment with Arvinas at the time of this work.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. . Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res 2015;21:2315–24. - PubMed
-
- Robinson D, Van Allen EM, Wu Y-M, Schultz N, Lonigro RJ, Mosquera J-M, et al. . Integrative clinical genomics of advanced prostate cancer. Cell 2015;162:454. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

