A Putative Frizzled 7-Targeting Compound Acts as a Firefly Luciferase Inhibitor
- PMID: 39670643
- PMCID: PMC11684006
- DOI: 10.1021/acs.jmedchem.4c02766
A Putative Frizzled 7-Targeting Compound Acts as a Firefly Luciferase Inhibitor
Abstract
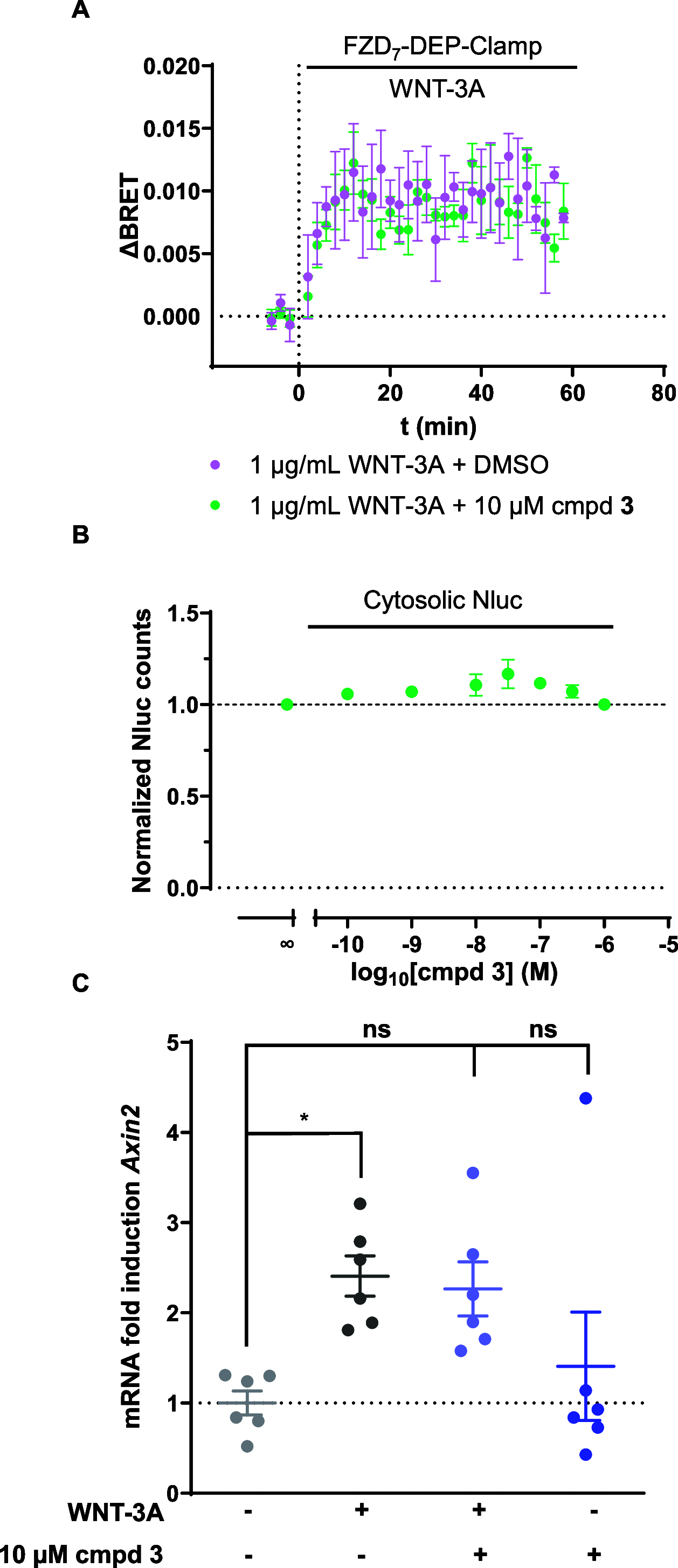
The Frizzled family (FZD1-10) of G protein-coupled receptors regulates WNT signaling mediating proliferative input. Dysregulation of FZD7 and exaggerated WNT/β-catenin signaling is frequently observed in intestinal cancers. Therefore, it is attractive to develop therapeutics targeting FZD7 for cancer treatment. Structure-based virtual screening has identified compound 28, which inhibited WNT/β-catenin signaling based on the luciferase-based reporter gene TOPFlash assay. However, upon pharmacological validation, compound 28 rather acts as a potent Firefly luciferase (Fluc) inhibitor (IC50 = 30 nM), matching the reported IC50 for compound 28-mediated inhibition in the TOPFlash assay. Moreover, we employed Fluc-independent assays, a FZD7-focused bioluminescence resonance energy transfer biosensor and quantitative PCR, to emphasize the inability of compound 28 to inhibit the WNT-3A-induced conformational dynamics in FZD7 and transcription of Axin2, a WNT target gene. Thus, we underline the importance of counter screens to validate compounds that interfere with the detection technology used for compound screening.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

