Developing, Characterizing, and Modeling CRISPR-Based Point-of-Use Pathogen Diagnostics
- PMID: 39670656
- PMCID: PMC11744932
- DOI: 10.1021/acssynbio.4c00469
Developing, Characterizing, and Modeling CRISPR-Based Point-of-Use Pathogen Diagnostics
Abstract
Recent years have seen intense interest in the development of point-of-care nucleic acid diagnostic technologies to address the scaling limitations of laboratory-based approaches. Chief among these are combinations of isothermal amplification approaches with CRISPR-based detection and readouts of target products. Here, we contribute to the growing body of rapid, programmable point-of-care pathogen tests by developing and optimizing a one-pot NASBA-Cas13a nucleic acid detection assay. This test uses the isothermal amplification technique NASBA to amplify target viral nucleic acids, followed by the Cas13a-based detection of amplified sequences. We first demonstrate an in-house formulation of NASBA that enables the optimization of individual NASBA components. We then present design rules for NASBA primer sets and LbuCas13a guide RNAs for the fast and sensitive detection of SARS-CoV-2 viral RNA fragments, resulting in 20-200 aM sensitivity. Finally, we explore the combination of high-throughput assay condition screening with mechanistic ordinary differential equation modeling of the reaction scheme to gain a deeper understanding of the NASBA-Cas13a system. This work presents a framework for developing a mechanistic understanding of reaction performance and optimization that uses both experiments and modeling, which we anticipate will be useful in developing future nucleic acid detection technologies.
Keywords: CRISPR-Cas; NASBA; ODE modeling; POC pathogen tests.
Conflict of interest statement
The authors declare the following competing financial interest(s): K.K.A., M.C.J., and J.B.L. are founders and have financial interest in Stemloop, Inc., and these interests are reviewed and managed by Northwestern University and Stanford University in accordance with their conflict-of-interest policies.
Figures

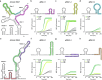


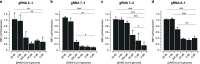
Update of
-
Developing, characterizing and modeling CRISPR-based point-of-use pathogen diagnostics.bioRxiv [Preprint]. 2024 Jul 3:2024.07.03.601853. doi: 10.1101/2024.07.03.601853. bioRxiv. 2024. Update in: ACS Synth Biol. 2025 Jan 17;14(1):129-147. doi: 10.1021/acssynbio.4c00469. PMID: 39005318 Free PMC article. Updated. Preprint.
References
-
- Gootenberg J. S.; Abudayyeh O. O.; Lee J. W.; Essletzbichler P.; Dy A. J.; Joung J.; Verdine V.; Donghia N.; Daringer N. M.; Freije C. A.; Myhrvold C.; Bhattacharyya R. P.; Livny J.; Regev A.; Koonin E. V.; Hung D. T.; Sabeti P. C.; Collins J. J.; Zhang F. Nucleic Acid Detection with CRISPR-Cas13a/C2c2. Science 2017, 356 (6336), 438–442. 10.1126/science.aam9321. - DOI - PMC - PubMed
-
- Joung J.; Ladha A.; Saito M.; Kim N.-G.; Woolley A. E.; Segel M.; Barretto R. P. J.; Ranu A.; Macrae R. K.; Faure G.; Ioannidi E. I.; Krajeski R. N.; Bruneau R.; Huang M.-L. W.; Yu X. G.; Li J. Z.; Walker B. D.; Hung D. T.; Greninger A. L.; Jerome K. R.; Gootenberg J. S.; Abudayyeh O. O.; Zhang F. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383 (15), 1492–1494. 10.1056/NEJMc2026172. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

