Novel broadly reactive monoclonal antibody protects against Pseudomonas aeruginosa infection
- PMID: 39670709
- PMCID: PMC11784295
- DOI: 10.1128/iai.00330-24
Novel broadly reactive monoclonal antibody protects against Pseudomonas aeruginosa infection
Abstract
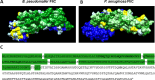
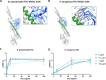
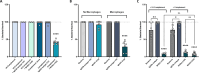
The incidence of infections attributed to antimicrobial-resistant (AMR) pathogens has increased exponentially over the recent decades reaching 1.27 million deaths worldwide in 2019. Without intervention, these infections are predicted to cause up to 10 million deaths a year and incur costs of up to 100 trillion US dollars globally by 2050. The emergence of AMR bacteria such as the ESKAPEE pathogens, and in particular Pseudomonas aeruginosa and species from the genus Burkholderia, underscores an urgent need for new therapeutic strategies. Monoclonal antibody (mAb) therapy offers a promising alternative to treat and prevent bacterial infections. In this study, we used peptides from highly conserved areas of the bacterial flagellin to generate monoclonal antibodies capable of broad binding to flagellated Gram-negative bacteria. We generated a broadly reactive IgG2bĸ mAb (WVDC-2109) that recognizes P. aeruginosa, Burkholderia sp., and other Gram-negative pathogens of interest. Characterization of the therapeutic potential of this antibody was determined using P. aeruginosa as model. In vitro characterization of WVDC-2109 demonstrated complement-mediated bactericidal activity and enhanced opsonophagocytosis of P. aeruginosa. Prophylactic administration of WVDC-2109 markedly improved survival and outcome in a lethal sepsis model and a sub-lethal murine pneumonia model of P. aeruginosa infection, reducing bacterial burden and inflammation. These findings suggest that WVDC-2109 and similar FliC-targeting antibodies could be valuable in preventing or treating diseases caused by P. aeruginosa as well as other life-threatening diseases of concern.IMPORTANCEAntimicrobial resistance (AMR) costs hundreds of thousands of lives and billions of dollars annually. To protect the population against these infections, it is imperative to develop new medical countermeasures targeting AMR pathogens like P. aeruginosa and Burkholderia sp. The administration of broadly reactive monoclonal antibodies can represent an alternative to treat and prevent infections caused by multi-drug-resistant bacteria. Unlike vaccines, antibodies can provide protection regardless of the immune status of the infected host. In this study, we generated an antibody capable of recognizing flagellin from P. aeruginosa and B. pseudomallei along with other Gram-negative pathogens of concern. Our findings demonstrate that the administration of the monoclonal antibody WVDC-2109 enhances survival rates and outcomes in different murine models of P. aeruginosa infection. These results carry significant implications in the field given that there are no available vaccines for P. aeruginosa.
Keywords: Burkholderia; Pseudomonas aeruginosa; anti-microbial resistance; monoclonal antibodies.
Conflict of interest statement
M.M.-B. and M.B. are inventors on a pending patent application related to the sequence of WVDC-2109. The remaining authors declare no competing interests.
Figures


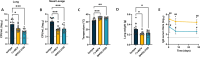



References
-
- Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. 2018. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 7:79. doi: 10.1186/s13756-018-0370-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

