The Campylobacter jejuni BumS sensor phosphatase detects the branched short-chain fatty acids isobutyrate and isovalerate as direct cues for signal transduction
- PMID: 39670710
- PMCID: PMC11796366
- DOI: 10.1128/mbio.03278-24
The Campylobacter jejuni BumS sensor phosphatase detects the branched short-chain fatty acids isobutyrate and isovalerate as direct cues for signal transduction
Abstract
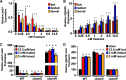
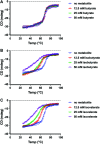
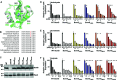
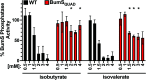
Two-component signal transduction systems (TCSs) are nearly ubiquitous across bacterial species and enable bacteria to sense and respond to specific cues for environmental adaptation. The Campylobacter jejuni BumSR TCS is unusual in that the BumS sensor exclusively functions as a phosphatase rather than a kinase to control phosphorylated levels of its cognate BumR response regulator (P-BumR). We previously found that BumSR directs a response to the short-chain fatty acid butyrate generated by resident microbiota so that C. jejuni identifies ideal lower intestinal niches in avian and human hosts for colonization. However, butyrate is an indirect cue for BumS and did not inhibit in vitro BumS phosphatase activity for P-BumR. In this work, we expanded the repertoire of lower intestinal metabolites that are cues sensed by BumS that modulate the expression of genes required for colonization to include the branched short-chain fatty acids isobutyrate and isovalerate. Unlike butyrate, isobutyrate and isovalerate inhibited in vitro BumS phosphatase activity for P-BumR, indicating that these metabolites are direct cues for BumS. Isobutyrate and isovalerate reduced the thermostability of BumS and caused a reorganization of protein structure to suggest how sensing these cues inhibits phosphatase activity. We also identified residues in the BumS sensory domain required to detect isobutyrate, isovalerate, and butyrate and for optimal colonization of hosts to reveal how gut bacteria can recognize these intestinal metabolites. Our work reveals how this unusual bacterial sensor phosphatase senses a repertoire of intestinal metabolites and how cues alter BumSR signal transduction to influence C. jejuni colonization of hosts.IMPORTANCETCSs are prevalent in many bacteria, but the cues sensed by each are not actually known for many of these systems. Microbiota-generated butyrate in human and avian hosts is detected by the Campylobacter jejuni BumS sensor phosphatase so that the bacterium identifies ideal lower intestinal niches for colonization. However, BumS only indirectly senses butyrate to inhibit dephosphorylation of its cognate BumR response regulator. Here, we expanded the repertoire of cues sensed by BumS to the branched-short chain fatty acids isobutyrate and isovalerate that are also abundant in the lower intestines. Both isobutyrate and isovalerate are potent, direct cues for BumS, whereas butyrate is an indirect cue. Leveraging isobutyrate and isovalerate as direct cues, we reveal BumS structure is altered upon cue detection to inhibit its phosphatase activity. We provide an understanding of the mechanics of an unusual mode of signal transduction executed by BumSR and other bacterial sensor phosphatase-driven TCSs.
Keywords: BumS; Campylobacter jejuni; butyrate; isobutyrate; isovalerate; sensor phosphatase; two-component signal transduction system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- R01 HD095830/HD/NICHD NIH HHS/United States
- R01AI065539/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 3R01HD095830-S1/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R35GM131760/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01HD095830/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources

