FSTL1 and TLR4 interact with PEDV structural proteins to promote virus adsorption to host cells
- PMID: 39670742
- PMCID: PMC11784190
- DOI: 10.1128/jvi.01837-24
FSTL1 and TLR4 interact with PEDV structural proteins to promote virus adsorption to host cells
Abstract
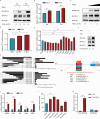
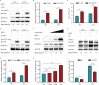
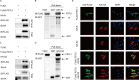
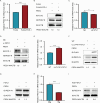
Infection with porcine epidemic diarrhea virus (PEDV) results in enormous economic damage to the global swine industry. PEDV starts its life cycle by binding to the receptors of host cells and adsorbing onto the cellular surfaces. However, it is still unknown how PEDV adsorbs onto the surface of host cells and the mechanism beneath the interplay of host cell transmembrane protein with PEDV proteins. FSTL1, which is a secreted glycoprotein, participates in diverse pathological and physiological processes, including immune modulation and cell proliferation and differentiation. The transmembrane protein, TLR4, serves as a pattern recognition receptor recognizing a broad spectrum of pathogens, which exerts a crucial effect on the host immune system. In this study, we identified that FSTL1 promoted PEDV infection. Further studies demonstrated the interactive relationship between FSTL1 and PEDV structural proteins (N and S2). In addition, we also confirmed that TLR4 interacted with FSTL1 and PEDV N, S1, and S2 proteins on the cell surface. Moreover, FSTL1 promoted the interaction of TLR4 and PEDV and induced viral adsorption to host cells. This study offers explicit evidence that FSTL1 and TLR4 act as mediators for host cell adsorption of PEDV by interacting with PEDV N/S proteins.IMPORTANCEAs a highly infectious porcine epidemic diarrhea virus (PEDV)-induced intestinal condition of swine, porcine epidemic diarrhea (PED) results in a 100% death rate among suckling piglets and poses a serious economic burden to global swine farming. Therefore, it is essential to investigate the mechanism of virus infection, replication, and proliferation. Virus begins its life cycle by binding to the receptor of host cells and adsorbing onto the cellular surfaces. However, it remains unclear how PEDV adsorbs onto the host cell surfaces. This study revealed that host protein FSTL1 interacted with the PEDV N and S2 proteins, while TLR4 interacted with the FSTL1 and PEDV proteins (N, S1, and S2). Moreover, we thoroughly and methodically demonstrated that FSTL1 was engaged in the PEDV internalization and attachment processes by promoting the recognition of PEDV N\S proteins by TLR4 and induced the viral adsorption to host cells.
Keywords: FSTL1; PEDV; TLR4; structural proteins; virus adsorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
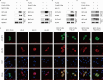
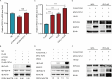

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

