Arginase-II gene deficiency reduces skeletal muscle aging in mice
- PMID: 39670851
- PMCID: PMC11723659
- DOI: 10.18632/aging.206173
Arginase-II gene deficiency reduces skeletal muscle aging in mice
Abstract
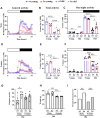
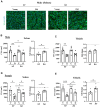
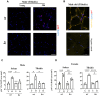
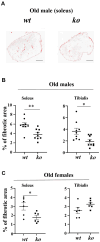
Age-associated sarcopenia decreases mobility and is promoted by cell senescence, inflammation, and fibrosis. The mitochondrial enzyme arginase-II (Arg-II) plays a causal role in aging and age-associated diseases. Therefore, we aim to explore the role of Arg-II in age-associated decline of physical activity and skeletal muscle aging in a mouse model. Young (4-6 months) and old (20-24 months) wild-type (wt) mice and mice deficient in arg-ii (arg-ii-/-) of both sexes are investigated. We demonstrate a decreased physical performance of old wt mice, which is partially prevented in arg-ii-/- animals, particularly in males. The improved phenotype of arg-ii-/- mice in aging is associated with reduced sarcopenia, cellular senescence, inflammation, and fibrosis, whereas age-associated decline of microvascular endothelial cell density, satellite cell numbers, and muscle fiber types in skeletal muscle is prevented in arg-ii-/- mice. Finally, we demonstrate an increased arg-ii gene expression level in aging skeletal muscle and found Arg-II protein expression in endothelial cells and fibroblasts, but not in skeletal muscle fibers, macrophages, and satellite cells. Our results suggest that increased Arg-II in non-skeletal muscle cells promotes age-associated sarcopenia, particularly in male mice.
Keywords: aging; arginase-II; cellular senescence; fibrosis; physical activity; skeletal muscle.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

