pERK transition-induced directional mode switching promotes epithelial tumor cell migration
- PMID: 39671185
- PMCID: PMC11665884
- DOI: 10.1073/pnas.2318871121
pERK transition-induced directional mode switching promotes epithelial tumor cell migration
Abstract
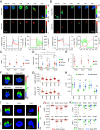
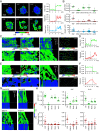
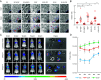
Increasing evidence suggests that tumor cells exhibit extreme plasticity in migration modes in order to adapt to microenvironments. However, the underlying mechanism for governing the migration mode switching is still unclear. Here, we revealed that epithelial tumor cells could develop a stable directional mode driven by hyperactivated ERK activity. This highly activated and dynamically changing ERK activity, called pERK transition, is crucial for inducing the switch from pauses state to directional movement and is also necessary for maintaining epithelial tumor cells in the directional mode. PERK transition integrated pERK surf, the dynamic and localized ERK activity at the leading edge. The sequential activation of RhoA and Rac1 by pERK transition played critical roles in generation of pERK surf activity through a movement feedback mechanism. PERK transition activity converted the orderly collective migration into the disordered dispersal movement, enhanced the invasiveness of epithelial tumor cells, and promoted their metastasis in immune-deficient mice. These findings revealed that the exquisite spatiotemporal organization of ERK activity orchestrates migration and invasion of tumor cells and provide evidence for the mechanism underlying migration mode switching in epithelial tumor cells.
Keywords: migration mode switching; migration plasticity; pERK surf; pERK transition; rampage mode.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Chaffer C. L., Weinberg R. A., A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011). - PubMed
-
- Jain S., Ladoux B., Mege R. M., Mechanical plasticity in collective cell migration. Curr. Opin. Cell Biol. 72, 54–62 (2021). - PubMed
-
- Nishimura K., et al. , Regulation of invasive potential of human prostate cancer cell lines by hepatocyte growth factor. Int. J. Urol. 5, 276–281 (1998). - PubMed
-
- Liu Y. J., et al. , Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015). - PubMed
-
- Lehmann S., et al. , Hypoxia induces a HIF-1-dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr. Biol. 27, 392–400 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

