Estimated Vaccine Effectiveness for Respiratory Syncytial Virus-Related Lower Respiratory Tract Disease
- PMID: 39671195
- PMCID: PMC11645642
- DOI: 10.1001/jamanetworkopen.2024.50832
Estimated Vaccine Effectiveness for Respiratory Syncytial Virus-Related Lower Respiratory Tract Disease
Erratum in
-
Error in Main Text, Figures, Tables, and Supplement.JAMA Netw Open. 2025 Jun 2;8(6):e2523111. doi: 10.1001/jamanetworkopen.2025.23111. JAMA Netw Open. 2025. PMID: 40553477 Free PMC article. No abstract available.
Abstract
Importance: Clinical trials have demonstrated high vaccine efficacy (VE) against lower respiratory tract disease (LRTD) but enrolled a smaller proportion of persons aged 75 years or older and those with comorbidities than seen in highest-risk populations in clinical practice settings. Additionally, VE against respiratory syncytial virus (RSV)-related hospitalizations and emergency department (ED) visits is not yet fully described.
Objective: To estimate Respiratory Syncytial Virus Prefusion F (RSVpreF) effectiveness in older adults.
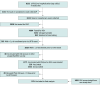
Design, setting, and participants: This was a retrospective case-control study with a test negative design. Cases were adults aged 60 years or older with hospitalizations or ED visits at Kaiser Permanente of Southern California for LRTD from November 24, 2023, to April 9, 2024, who had respiratory swabs collected and tested for RSV. Two control definitions were prespecified: (1) strict controls included RSV-negative LRTD events that were negative for human metapneumovirus, SARS-CoV-2, and influenza, and positive for a nonvaccine preventable cause (primary) and (2) broad controls included all RSV-negative LRTD events (sensitivity analysis). Enhanced specimen collection was conducted to salvage clinical respiratory swabs not tested for RSV during routine care. Data were analyzed from May to September 2024.
Exposure: RSVpreF vaccine receipt during the first RSV season after licensure and 21 or more days before LRTD event.
Main outcomes and measures: Estimated VE against first episode of RSV-related LRTD hospitalization or ED visit.
Results: A total of 7047 LRTD-related hospitalizations or ED encounters with RSV testing results were included. The mean (SD) age was 76.8 (9.6) years; 3819 (54.2%) were female; 839 (11.9%) were non-Hispanic Asian or Pacific Islander, 2323 (33.0%) were Hispanic, 1197 (17.0%) were non-Hispanic Black, and 2602 (36.9%) were non-Hispanic White; 998 (14.2%) were immunocompromised; and 6573 (93.3%) had 1 or more Charlson comorbidity. Using strict controls, estimated adjusted VE was 91% (95% CI, 59%-98%). Using broad controls, estimated adjusted VE was 90% (95% CI, 59%-97%).
Conclusions and relevance: In a high-risk, general population, RSVpreF vaccination conferred protection against RSV-related LRTD in the hospital and ED settings among US adults aged 60 years or older, the majority of whom were aged 75 years or older and had comorbidities. These data support use of this vaccine in older adults.
Conflict of interest statement
Figures
References
-
- Woodruff RC, Melgar M, Pham H, et al. ; Respiratory Syncytial Virus Hospitalization Surveillance Network (RSV-NET) . Acute cardiac events in hospitalized older adults with respiratory syncytial virus infection. JAMA Intern Med. 2024;184(6):602-611. doi: 10.1001/jamainternmed.2024.0212 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

