Primary Care-Based Digital Health-Enabled Stroke Management Intervention: Long-Term Follow-Up of a Cluster Randomized Clinical Trial
- PMID: 39671199
- PMCID: PMC11645652
- DOI: 10.1001/jamanetworkopen.2024.49561
Primary Care-Based Digital Health-Enabled Stroke Management Intervention: Long-Term Follow-Up of a Cluster Randomized Clinical Trial
Abstract
Importance: Despite evidence of the short-term benefits of multicomponent primary care-based interventions, their long-term effects are unproven.
Objective: To evaluate the long-term outcomes of a system-integrated technology-enabled model of care (SINEMA intervention) for stroke management for systolic blood pressure (BP) and other outcomes among patients with stroke in China.
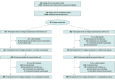
Design, setting, and participants: This long-term follow-up included community-dwelling clinically stable surviving participants with stroke in an open-label cluster randomized clinical trial. Of 218 villages from Nanhe County in Hebei, China, an area with suboptimal health care resources and stroke prevalence doubling the national average, 50 villages (clusters) were recruited between June 23 and July 29, 2017, and randomized in a 1:1 ratio to an intervention or a control arm (usual care). The intervention lasted 1 year (to July 31, 2018), with a posttrial observational follow-up conducted from October 1, 2022, to August 27, 2023.
Interventions: Village doctors were provided with training, performance-based incentives, technical support, and customized mobile health tools to deliver monthly follow-up to patients. Patients also received daily voice messages emphasizing medication adherence and physical activity. No intervention was requested or supported during the posttrial period.
Main outcomes and measures: Between-arm differences in intention-to-treat analyses of individual-level changes from baseline to long-term posttrial in systolic BP (primary outcome) and stroke recurrence, diastolic BP, BP control, antihypertensive medication use and regimen adherence, and disability (secondary outcomes).
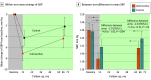
Results: Among a total of 1042 stroke survivors, 44 (4.2%) were lost to follow-up and 998 (mean [SD] age at baseline: 65.0 [8.2] years; 544 [54.4%] men) completed posttrial assessment at a mean (SD) period of 66.6 (3.7) months (5.5 years) after baseline. The multicomponent intervention was associated with an estimated between-arm net reduction in systolic BP of -2.8 (95% CI, -5.3 to -0.3) mm Hg (P = .03). Most secondary outcomes showed a tendency toward lasting effects, with a notable absolute net reduction of 6.0 (95% CI, -11.3 to -0.7) percentage points and risk ratio of 0.77 (95% CI, 0.61-0.99) for stroke recurrence. In subgroup analyses, significant between-arm differences were observed among women and people with lower educational attainment, lower income, and higher use of and adherence to medications.
Conclusions and relevance: In this long-term follow-up of a cluster randomized clinical trial, the 1-year intervention was associated with significantly reduced systolic BP and stroke recurrence at 5.5 years, providing evidence of long-term health and inequity-reducing benefits and holding promise for scaling up of the intervention in resource-limited settings.
Trial registration: ClinicalTrials.gov Identifier: NCT05792618.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

