TLR3 Knockdown Attenuates Pressure-Induced Neuronal Damage In Vitro
- PMID: 39671271
- PMCID: PMC11640903
- DOI: 10.1111/jcmm.70276
TLR3 Knockdown Attenuates Pressure-Induced Neuronal Damage In Vitro
Abstract
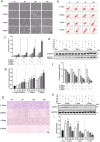
The disruption of nerve parenchyma and axonal networks triggered by spinal cord injury (SCI) can initiate a cascade of events associated with secondary injury. Toll-like receptors play a critical role in initiating and regulating immune-inflammatory responses following SCI; however, the precise involvement of Toll-like receptor-3 (TLR3) in secondary neuronal injury remains incompletely understood. To investigate the potential contribution of TLR3 in mediating neuronal pressure-induced damage, we established a stress-induced neuronal damage model using rat anterior horn motor neuron line (VSC4.1), which was subjected to varying levels and durations of sustained pressure. Our findings suggest that pressure induces neuronal damage and apoptosis, and reduced proliferation rates in VSC4.1 cells. Furthermore, this pressure-induced neuronal injury is accompanied by upregulation of TLR3 expression and activation of downstream TLR3 signalling molecules. Knockdown experiments targeting TLR3 significantly alleviate pressure-induced motor neuron injury and apoptosis within the anterior horn region while promoting mitochondria-related autophagy and reducing mitochondrial dysfunction via the TLR3/IRF3 and TLR3/NF-κB pathways.
Keywords: TLR3; apoptosis; autophagy; microtubule‐associated protein‐2; mitochondria; pressure‐injured; spinal cord injury.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Yao M., Pu P. M., Li Z. Y., et al., “Melatonin Restores Endoplasmic Reticulum Homeostasis to Protect Injured Neurons in a Rat Model of Chronic Cervical Cord Compression,” Journal of Pineal Research 74, no. 4 (2023): e12859. - PubMed
-
- Fakhri S., Sabouri S., Kiani A., et al., “Intrathecal Administration of Naringenin Improves Motor Dysfunction and Neuropathic Pain Following Compression Spinal Cord Injury in Rats: Relevance to Its Antioxidant and Anti‐Inflammatory Activities,” Korean Journal of Pain 35, no. 3 (2022): 291–302. - PMC - PubMed
-
- Farooque M., “Spinal Cord Compression Injury in the Mouse: Presentation of a Model Including Assessment of Motor Dysfunction,” Acta Neuropathologica 100, no. 1 (2000): 13–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

