Ex vivo expansion and hydrogel-mediated in vivo delivery of tissue-resident memory T cells for immunotherapy
- PMID: 39671478
- PMCID: PMC11641059
- DOI: 10.1126/sciadv.adm7928
Ex vivo expansion and hydrogel-mediated in vivo delivery of tissue-resident memory T cells for immunotherapy
Erratum in
-
Erratum for the Research Article: "Ex vivo expansion and hydrogel-mediated in vivo delivery of tissue-resident memory T cells for immunotherapy" by S. Li et al.Sci Adv. 2025 Mar 21;11(12):eadx1775. doi: 10.1126/sciadv.adx1775. Epub 2025 Mar 21. Sci Adv. 2025. PMID: 40117379 Free PMC article. No abstract available.
Abstract
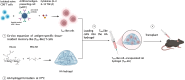
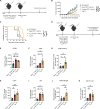
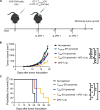
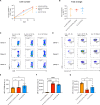
Tissue-resident memory T (TRM) cells preferentially reside in peripheral tissues, serving as key players in tumor immunity and immunotherapy. The lack of effective approaches for expanding TRM cells and delivering these cells in vivo hinders the exploration of TRM cell-mediated cancer immunotherapy. Here, we report a nanoparticle artificial antigen-presenting cell (nano-aAPC) ex vivo expansion approach and an in vivo delivery system for TRM cells. Using the nano-aAPC platform, we expanded functional antigen-specific murine and human TRM-like CD8+ T cells ex vivo. We also developed an injectable macroporous hyaluronic acid (HA) hydrogel to deliver TRM-like cells. TRM-like cells delivered in the optimized HA hydrogel trigger robust local and systemic antitumor immunity and show synergistic effects with anti-PD-1 treatment. Our findings suggest that nano-aAPC-induced TRM-like cells, coupled with a hydrogel delivery system, offer an efficient way to advance the understanding of TRM cell-mediated cancer therapy.
Figures



References
-
- Amsen D., van Gisbergen K. P. J. M., Hombrink P., van Lier R. A. W., Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19, 538–546 (2018). - PubMed
-
- Byrne A., Savas P., Sant S., Li R., Virassamy B., Luen S. J., Beavis P. A., Mackay L. K., Neeson P. J., Loi S., Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat. Rev. Clin. Oncol. 17, 341–348 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

