Predicting optimal mixotrophic metabolic strategies in the global ocean
- PMID: 39671489
- PMCID: PMC11641107
- DOI: 10.1126/sciadv.adr0664
Predicting optimal mixotrophic metabolic strategies in the global ocean
Abstract
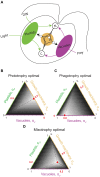
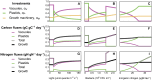
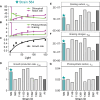
Mixotrophic protists combine photosynthesis with the ingestion of prey to thrive in resource-limited conditions in the ocean. Yet, how they fine-tune resource investments between their two different metabolic strategies remains unclear. Here, we present a modeling framework (Mixotroph Optimal Contributions to Heterotrophy and Autotrophy) that predicts the optimal (growth-maximizing) investments of carbon and nitrogen as a function of environmental conditions. Our model captures a full spectrum of trophic modes, in which the optimal investments reflect zero-waste solutions (i.e., growth is colimited by carbon and nitrogen) and accurately reproduces experimental results. By fitting the model to data for Ochromonas, we were able to predict metabolic strategies at a global scale. We find that high phagotrophic investment is the dominant strategy across different oceanic biomes, used primarily for nitrogen acquisition. Our results therefore support empirical observations of the importance of mixotrophic grazers to upper ocean bacterivory.
Figures

References
-
- Worden A. Z., Follows M. J., Giovannoni S. J., Wilken S., Zimmerman A. E., Keeling P. J., Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 347, 1257594 (2015). - PubMed
-
- Selosse M.-A., Charpin M., Not F., Mixotrophy everywhere on land and in water: The grand écart hypothesis. Ecol. Lett. 20, 246–263 (2017). - PubMed
-
- Crane K. W., Grover J. P., Coexistence of mixotrophs, autotrophs, and heterotrophs in planktonic microbial communities. J. Theor. Biol. 262, 517–527 (2010). - PubMed
-
- Mitra A., Flynn K. J., Burkholder J. M., Berge T., Calbet A., Raven J. A., Granéli E., Glibert P. M., Hansen P. J., Stoecker D. K., Thingstad F., Tillmann U., Våge S., Wilken S., Zubkov M. V., The role of mixotrophic protists in the biological carbon pump. Biogeosciences 11, 995–1005 (2014).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

