Regulation of volume-regulated anion channels alters sensitivity to platinum chemotherapy
- PMID: 39671496
- PMCID: PMC11641020
- DOI: 10.1126/sciadv.adr9364
Regulation of volume-regulated anion channels alters sensitivity to platinum chemotherapy
Abstract
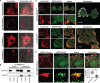
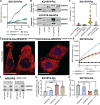
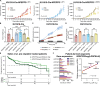
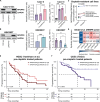
Cisplatin-based chemotherapy is used across many common tumor types, but resistance reduces the likelihood of long-term survival. We previously found the puromycin-sensitive aminopeptidase, NPEPPS, as a druggable driver of cisplatin resistance in vitro and in vivo and in patient-derived organoids. Here, we present a general mechanism where NPEPPS interacts with the volume-regulated anion channels (VRACs) to control cisplatin import into cells and thus regulate cisplatin response across a range of cancer types. We also find the NPEPPS/VRAC gene expression ratio is a predictive measure of cisplatin response in multiple cancer cohorts, showing the broad applicability of this mechanism. Our work describes a specific mechanism of cisplatin resistance, which, given the characteristics of NPEPPS as a drug target, has the potential to improve cancer patient outcomes. In addition, we describe an intracellular mechanism regulating VRAC activity, which is critical for volume regulation in normal cells - a finding with functional implications beyond cancer.
Figures


References
-
- Aldossary S. A., Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 12, 7–15 (2019).
-
- Alassadi S., Pisani M. J., Wheate N. J., A chemical perspective on the clinical use of platinum-based anticancer drugs. Dalton Trans. 51, 10835–10846 (2022). - PubMed
-
- Galanski M. S., Jakupec M. A., Keppler B. K., Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 12, 2075–2094 (2005). - PubMed
-
- Xiao X., Oswald J. T., Wang T., Zhang W., Li W., Use of anticancer platinum compounds in combination therapies and challenges in drug delivery. Curr. Med. Chem. 27, 3055–3078 (2020). - PubMed
-
- van der Heijden M. S., Sonpavde G., Powles T., Necchi A., Burotto M., Schenker M., Sade J. P., Bamias A., Beuzeboc P., Bedke J., Oldenburg J., Chatta G., Ürün Y., Ye D., He Z., Valderrama B. P., Ku J. H., Tomita Y., Filian J., Wang L., Purcea D., Patel M. Y., Nasroulah F., Galsky M. D., CheckMate 901 Trial Investigators , Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 389, 1778–1789 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

