SWOG S1815: A Phase III Randomized Trial of Gemcitabine, Cisplatin, and Nab-Paclitaxel Versus Gemcitabine and Cisplatin in Newly Diagnosed, Advanced Biliary Tract Cancers
- PMID: 39671534
- PMCID: PMC11798714
- DOI: 10.1200/JCO-24-01383
SWOG S1815: A Phase III Randomized Trial of Gemcitabine, Cisplatin, and Nab-Paclitaxel Versus Gemcitabine and Cisplatin in Newly Diagnosed, Advanced Biliary Tract Cancers
Abstract
Purpose: SWOG S1815 was a randomized, open label phase III trial, evaluating gemcitabine, nab-paclitaxel, and cisplatin (GAP) versus gemcitabine and cisplatin (GC) in patients with newly diagnosed advanced biliary tract cancers (BTCs).
Methods: Patients with newly diagnosed locally advanced unresectable or metastatic BTC, including intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) and gallbladder carcinoma (GBC), were randomly assigned 2:1 to either GAP (gemcitabine 800 mg/m2, cisplatin 25 mg/m2, and nab-paclitaxel 100 mg/m2 intravenously once per day on days 1 and 8 of a 21-day cycle) or GC (gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 intravenously once per day on days 1 and 8 of a 21-day cycle).
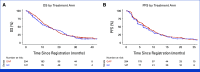
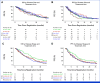
Results: Among 452 randomly assigned participants, 441 were eligible and analyzable, 67% with ICC, 16% with GBC, and 17% with ECC. There was no significant difference in overall survival (OS) between GAP versus GC. Median OS with GAP was 14.0 months (95% CI, 12.4 to 16.1) and 13.6 months with GC (95% CI, 9.7 to 16.6); hazard ratio (HR), 0.91 (95% CI, 0.72 to 1.14); P = .41. Median progression-free survival (PFS) was similar between groups with median PFS for GAP being 7.5 months (95% CI, 6.4 to 8.5) versus 6.3 months for GC (95% CI, 4.4 to 8.2); HR, 0.89 (95% CI, 0.71 to 1.12); P = .32. In exploratory subset analyses, the OS and PFS benefits of GAP versus GC treatment were greater in locally advanced disease compared with metastatic disease, although not statistically significant (interaction P = .14 for OS and P = .17 for PFS). Moreover, GAP versus GC showed greater improvement in PFS among participants with GBC than those with ICC or ECC (interaction P = .01), but not OS (interaction P = .28).
Conclusion: The addition of a taxane in the GAP regimen to the standard gemcitabine-cisplatin regimen did not improve OS in newly diagnosed BTC. More toxicity was encountered with GAP versus GC.
Trial registration: ClinicalTrials.gov NCT03768414.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Valle JW, Kelley RK, Nervi B, et al. : Biliary tract cancer. Lancet 397:428-444, 2021 - PubMed
-
- Kelley RK, Ueno M, Yoo C, et al. : Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401:1853-1865, 2023 - PubMed
-
- Oh D-Y, Ruth He A, Qin S, et al. : Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid 1:EVIDoa2200015, 2022 - PubMed
-
- Wei D, Cheng X, Du C, et al. : Stroma-targeted nanoparticles that remodel stromal alignment to enhance drug delivery and improve the antitumor efficacy of nab-paclitaxel in pancreatic ductal adenocarcinoma models. Nano Today 45:101533, 2022
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

