Employing artificial intelligence for optimising antibiotic dosages in sepsis on intensive care unit: a study protocol for a prospective observational study (KI.SEP)
- PMID: 39672586
- PMCID: PMC11647398
- DOI: 10.1136/bmjopen-2024-086094
Employing artificial intelligence for optimising antibiotic dosages in sepsis on intensive care unit: a study protocol for a prospective observational study (KI.SEP)
Abstract
Introduction: In sepsis treatment, achieving and maintaining effective antibiotic therapy is crucial. However, optimal antibiotic dosing faces challenges due to significant variability among patients with sepsis. Therapeutic drug monitoring (TDM), the current gold standard, lacks initial dosage adjustments and global availability. Even with daily TDM, antibiotic serum concentrations (ASCs) often deviate from the therapeutic range. This study addresses these challenges by developing machine learning (ML)-based ASC prediction models capable of handling variable data input and encompassing diverse clinical, laboratory, microbiological and proteomic parameters without the need for daily TDM.
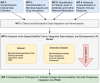
Methods: This prospective observational study is conducted in a German university hospital intensive care unit. Eligible sepsis patients receive continuous antibiotic therapy with piperacillin/tazobactam (n=100) or meropenem (n=100) within 24 hours. Exclusion criteria include refusal, pregnancy, lactation and severe anaemia (haemoglobin <8 g/dL). Blood samples for TDM are collected from patients, along with clinical and laboratory parameters on days 1-8 and day 30 or on discharge. ML models predicting ASC between day 1 and day 8 serve as primary and key secondary endpoints. We will use the collected data to develop multifaceted ML-based algorithms aimed at optimising antibiotic dosing in sepsis. Our two-way approach involves creating two distinct algorithms: the first focuses on predictive accuracy and generalisability using routine clinical parameters, while the second leverages an extended dataset including a plethora of factors currently insufficiently explored and not available in standard clinical practice but may help to enhance precision. Ultimately, these models are envisioned for integration into clinical decision support systems within patient data management systems, facilitating automated, personalised treatment recommendations for sepsis.
Ethics and dissemination: The study received approval from the Ethics Committee of the Medical Faculty of Ruhr-University Bochum (No. 23-7905). Findings will be disseminated through open-access publication in a peer-reviewed journal and social media channels.
Trial registration number: DRKS00032970.
Keywords: Artificial Intelligence; INTENSIVE & CRITICAL CARE; Machine Learning.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
