Epigenetic reprogramming in mouse and human primordial germ cells
- PMID: 39672813
- PMCID: PMC11671582
- DOI: 10.1038/s12276-024-01359-z
Epigenetic reprogramming in mouse and human primordial germ cells
Abstract
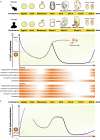
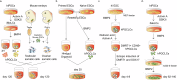
Primordial germ cells (PGCs) are the precursors of sperm and eggs. They undergo genome-wide epigenetic reprogramming to erase epigenetic memory and reset the genomic potential for totipotency. Global DNA methylation erasure is a crucial part of epigenetic resetting when DNA methylation levels decrease across the genome to <5%. However, certain localized regions exhibit slower demethylation or resistance to reprogramming. Since DNA methylation plays a crucial role in transcriptional regulation, this depletion in PGCs requires mechanisms independent of DNA methylation to regulate transcriptional control during PGC reprogramming. Histone modifications are predicted to compensate for the loss of DNA methylation in gene regulation. Different histone modifications exhibit distinct patterns in PGCs undergoing epigenetic programming at the genomic level during PGC development in conjunction with changes in DNA methylation. Together, they contribute to PGC-specific genomic regulation. Recent findings related to these processes provide a comprehensive overview of germline epigenetic reprogramming and its importance in mouse and human PGC development. Additionally, we evaluated the extent to which in vitro culture techniques have replicated the development processes of human PGCs.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

