Variants in the AGBL5 gene are responsible for autosomal recessive Retinitis pigmentosa with hearing loss
- PMID: 39672920
- PMCID: PMC12185745
- DOI: 10.1038/s41431-024-01768-8
Variants in the AGBL5 gene are responsible for autosomal recessive Retinitis pigmentosa with hearing loss
Abstract
The AGBL5 gene encodes for the Cytoplasmic Carboxypeptidase 5 (CCP5), an α-tubulin deglutamylase that cleaves the γ-carboxyl-linked branching point of glutamylated tubulin. To date, pathogenic variants in AGBL5 have been associated only with isolated retinitis pigmentosa (RP). Hearing loss has not been reported in AGBL5-caused retinal disease. In this study, we performed exome sequencing in probands of eight unrelated families from Italy, Spain, Palestine, Switzerland, and Greece. All subjects had a clinical diagnosis of (suspected) Usher syndrome type II for the concurrent presence of RP and post-verbal sensorineural hearing loss (SNHL) that ranged from mild to moderate.We identified biallelic sequence variants in AGBL5 in all analysed subjects. Four of the identified variants were novel. The variants co-segregated with the retinal and auditory phenotypes in additional affected family members. We did not detect any causative variants in known deafness or Usher syndrome genes that could explain the patients' hearing loss. We therefore conclude that SNHL is a feature of a syndromic presentation of AGBL5 retinopathy. This study provides the first evidence that mutations in AGBL5 can cause syndromic RP forms associated with hearing loss, probably due to dysfunction of sensory cilia in the retina and the inner ear.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: All procedures adhered to the tenets of the Declaration of Helsinki and were approved by the Ethics Committees of the participating institutes. An informed consent was obtained by all patients.
Figures

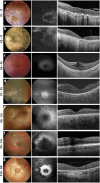

References
-
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. - PubMed
-
- Kastner S, Thiemann IJ, Dekomien G, Petrasch-Parwez E, Schreiber S, Akkad DA, et al. Exome sequencing reveals agbl5 as novel candidate gene and additional variants for retinitis pigmentosa in five turkish families. Invest Ophthalmol Vis Sci. 2015;56:8045–53. - PubMed
MeSH terms
Grants and funding
- PI22/00213/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- AC21_2/00022/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- FPU20/04736/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
LinkOut - more resources
Full Text Sources

