Improving Clinical Management of Diabetic Macular Edema: Insights from a Global Survey of Patients, Healthcare Providers, and Clinic Staff
- PMID: 39673039
- PMCID: PMC11724817
- DOI: 10.1007/s40123-024-01060-4
Improving Clinical Management of Diabetic Macular Edema: Insights from a Global Survey of Patients, Healthcare Providers, and Clinic Staff
Abstract
Introduction: In contrast with patients receiving therapy for retinal disease during clinical trials, those treated in routine clinical practice experience various challenges (including administrative, clinic, social, and patient-related factors) that can often result in high patient and clinic burden, and contribute to suboptimal visual outcomes. The objective of this study was to understand the challenges associated with clinical management of diabetic macular edema from the perspectives of patients, healthcare providers, and clinic staff, and identify opportunities to improve eye care for people with diabetes.
Methods: We conducted a survey of patients with diabetic macular edema, providers, and clinic staff in 78 clinics across 24 countries on six continents, representing a diverse range of individuals, healthcare systems, settings, and reimbursement models. Surveys comprised a series of single- and multiple-response questions completed anonymously. Data gathered included patient personal characteristics, challenges with appointment attendance, treatment experiences, and opportunities to improve support. Provider and clinic staff surveys asked similar questions about their perspectives; and clinic characteristics were also captured.
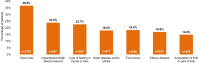
Results: Overall, 5681 surveys were gathered: 3752 from patients with diabetic macular edema, 680 from providers, and 1249 from clinic staff. Too many appointments, too short treatment intervals, difficulties in traveling to the clinic or arranging adequate support to travel, out-of-pocket costs, office/parking fees, and long waiting times were noted by all as contributing to increase the burden on the patient and caregiver. Patients generally desired more in-depth discussions with their provider, which would help with information exchange and better expectation-setting.
Conclusions: The wealth of systematic data generated by this global survey highlights the breadth and scale of challenges associated with the clinical management of patients with diabetic macular edema. Addressing the opportunities for improvement raised by patients, providers, and clinic staff could increase patient adherence to treatment, reduce appointment burden, and improve clinic capacity.
Keywords: Diabetes; Macular edema; Patient experience; Visual outcomes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Focke Ziemssen: Travel grants and personal fees: Allergan/AbbVie, Alimera, Bayer Healthcare, Biogen, Boehringer Ingelheim, Clearside, Janssen, Novartis, Novo Nordisk, Merck, Sharp & Dohme, Regeneron, Roche/Genentech, Sanofi, Sandoz, and Stada. Michelle Sylvanowicz: Employee: Bayer; Winfried M. Amoaku: Advisory board membership: AbbVie, Alcon, Alimera, Allergan, Apellis, Bayer, Bausch + Lomb, Bioeq, Novartis, and Pfizer; Speaker fees: Alimera, Allergan, Bayer, Novartis, and Pfizer; Support for travel: Alimera, Allergan, Bayer, Novartis, and Pfizer; Research sponsorship and funding: Allergan, Bayer, Boehringer Ingelheim, CenterVue, Gyroscope, Novartis, and Optos; Tariq Aslam: Tariq Aslam is an Editor in Chief of Ophthalmology and Therapy, and was not involved in the selection of peer reviewers for the manuscript nor any subsequent editorial decisions. Consultant: Bausch & Lomb, Bayer, Laboratoires Théa Pharmaceuticals, Novartis, Oraya, and Roche; Bora Eldem: Consultant: Allergan, Bayer, Novartis, and Roche; Robert P. Finger: Research grant: CentreVue, Heidelberg Engineering, Novartis, and Zeiss; Consultant: Alimera, Allergan, Bayer, Ellex, Inositec, Novartis, Opthea, Roche/Genentech, and Santhera; Support for travel: Novartis; Richard P. Gale: Consultant/advisory boards: Allergan, Alimera, Bayer, Novartis, and Santen; Educational travel grants: Allergan, Bayer, Heidelberg, and Novartis; Research grants: Allergan, Bayer, Novartis, and Roche; Laurent Kodjikian: Consultant: AbbVie, Alcon, Allergan, Bayer, Krystal Biotech, Novartis, Regeneron, and Théa; Jean-François Korobelnik: Consultant: AbbVie, Apellis, Bayer, Eyepoint Pharma, Ocuphire, Roche, Thea, Carl Zeiss Meditec; Member of DSMB for Alexion, Novo Nordisk, Opthea; Xiaofeng Lin: Consultant: Bayer; Anat Loewenstein: Consultant: 4DMT, AbbVie, Alkeus, Annexon, Apellis, Astellas, Bayer Health Care, Beyeonics, Eyepoint, Johnson & Johnson, NotalVision, Novartis, Ocular Therapeutics, Oculis, Ocuphire Pharma, Ocuterra, Opthea, Oxurion, Roche, Syneos; Paul Mitchell: Consultant: Allergan, Bayer, and Novartis; Steering Committee member: Bayer; Moira Murphy: Employee: Exploristics, Ltd.; David R. Owens: Consultant: Bayer; Nick Parker: Employee: The International Agency for the Prevention of Blindness; Ian Pearce: Lecture fees: Allergan, Bayer, Heidelberg Pharma, and Novartis; Consultant: Allergan, Alimera, Bayer, and Novartis, Support for travel: Allergan, Bayer, and Novartis; Francisco J. Rodríguez: Consultant: Bayer, Novartis, and Roche; Speaker: Bayer, Novartis, and Roche; Research funding: Novartis; Jude Stern: Employee: The International Agency for the Prevention of Blindness; S. James Talks: Advisory board member, speaker fees, and research support: Bayer and Novartis; Research grants: Boehringer Ingelheim and Roche; Consultant: Bayer; David T. Wong: Grants/research support: Bayer, Novartis, and Roche; Consultant: Alcon, Allergan, Apellis, Bausch Health, Bayer, Novartis, Roche, Topcon, and Zeiss; Equity: Arctic DX; Tien Yin Wong: Consulting fees/travel support/review fees: Aldropika Therapeutics, Bayer, Boehringer Ingelheim, Genentech, Iveric Bio, Novartis, Oxurion, Plano, Roche, Sanofi, Shanghai Henlius; Stock: EyRIS; Jane Barratt: Consultant: Bayer. Ethical Approval: The survey was developed according to guidelines from the Declaration of Helsinki and the World Health Organization’s International Ethical Guidelines for Biomedical Research. As a Primary Market Research Survey, ethics committee approvals are not required; however, local requirements were assessed by individual institutions and countries. Informed consent was acquired, no personally identifiable information was collected, and the survey did not inform treatment decisions.
Figures

References
-
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. - PubMed
-
- Leng T, Tripathy K, Bhagat N, et al. Diabetic macular edema. 2022. https://eyewiki.org/Diabetic_Macular_Edema. Accessed Aug 2024.
-
- GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–60. - PMC - PubMed
LinkOut - more resources
Full Text Sources

