Retrospective longitudinal study on the long-term impact of COVID-19 infection on polysomnographic evaluation in patients with Prader-Willi syndrome
- PMID: 39673054
- PMCID: PMC11639118
- DOI: 10.1186/s13023-024-03447-9
Retrospective longitudinal study on the long-term impact of COVID-19 infection on polysomnographic evaluation in patients with Prader-Willi syndrome
Abstract
Background: To evaluate the impact of coronavirus disease 2019 (COVID-19) on polysomnographic evaluation in patients with Prader-Willi syndrome (PWS).
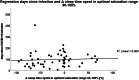
Patients and methods: A retrospective cohort study of two consecutive overnight polysomnograms (PSG) in 92 PWS patients (mean age 9.1, range 3.1-22 years). 57/92 participants (35 female) had a COVID-19 infection between the two consecutive examinations. 35 patients (21 female) had no infection (control group). Distribution of genetics was as follows: 13/57 (22.8%) deletion, 19/57 (33.3%) uniparental disomy, 2/57 (3,5%) imprinting defect, 3/57 (5.3%) non-deletion, 20/57 (35.1%) diagnosed by analyses of the methylation pattern of chromosome 15q11-13. Mean time interval between COVID-19 infection and post-COVID-19 evaluation was 96.2 days.
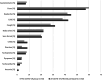
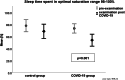
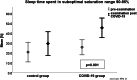
Results: Course of COVID-19 infection was asymptomatic 8/82 (9.8%), mild 63/82 (76.8%), medium 11/84 (13.4%). The five most frequently experienced symptoms in PWS patients were fever (56.1%); headache (45.1%); cold (42.7%); cough (31.7%) and body aches (21.95%). PWS patients who had COVID-19 infection had significantly lower mean oxygen saturation (SpO2) measured by pulse oximetry (post 94.8% vs. pre 95.7%, p = 0.001), lower detected lowermost SpO2 (post 86.2 vs. pre 87.3%, p = 0.003), and higher occurrence of hypopnoea (post 13.9 vs. pre 10.7, p = 0.001). Time in optimal SpO2 (95-100%) decreased significantly (post 54.3% vs. pre 73.8%, p = 0.001), whereas an increase was observed in time in suboptimal SpO2 (90-95%) (post 45.5% vs. 25.8%, p = 0.001) and in time in poor SpO2 (< 90%) (post 0.7% vs. pre 0.2%, p = 0.030). Body-Mass-Index (BMI)-SDS for PWS showed no differences between the groups at any time. BMI-SDS-differences showed no influence on differences in SpO2 evaluations. In the genetic subgroup with deletion there was a statistically significant effect on an increased number of OSA (p = 0.027). The genetic subgroup with uniparental disomy (UPD) was associated with a reduced risk of higher HF (p = 0.035) and less hypopnea (p = 0.011).
Conclusion: PWS patients predominantly experienced only mild to medium symptoms during COVID-19 infection without necessity of hospitalisation. However, on average three months after infection, differences in PSG evaluations were still apparent, manifesting in lower SpO2 and more frequent hypopnea. A long-lasting impairment of the pulmonary system due to the COVID-19 infection might be responsible.
Keywords: COVID-19; COVID-19 sequelae; Long COVID; Polysomnography; Prader-Willi syndrome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures performed in this study involving human participants were in accordance with the ethical standards of the University Hospital Bonn and with the 1964 Helsinki Declaration and its later amendments. Ethical approval was given by the institutional Ethics Committee of the University of Bonn, Germany (ethics committee number: 31/15; June 24, 2020). Consent for publication: All parents or guardians gave written informed consent for participation. Competing interests: The authors declare that they have no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

