Burden of Antimicrobial Resistance in Adult Hospitalized Patients With Cancer: A Multicenter Analysis
- PMID: 39673173
- PMCID: PMC11645461
- DOI: 10.1002/cam4.70495
Burden of Antimicrobial Resistance in Adult Hospitalized Patients With Cancer: A Multicenter Analysis
Abstract
Background: Infections are a leading cause of death in patients with cancer, but the proportion and rate of antimicrobial resistance (AMR) in hospitalized patients with cancer are not well understood.
Methods: This retrospective, cross-sectional evaluation of AMR assessed hospitalized adult patients in 168 United States (US) healthcare facilities between April 2018 and December 2022. Nonduplicate, noncontaminant Gram-negative and Gram-positive bacteria recovered from various samples (blood, respiratory, urine, etc.) were used to assess the rate of AMR pathogens per 1000 admissions and the proportion of AMR among bacterial isolates in patients with and without cancer.
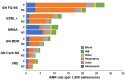
Findings: Among 4,612,620 admissions, 6.4% (297,500) were of patients with cancer and 93.6% (4,315,120) were of patients without cancer. AMR pathogen rates were higher in cancer patients than patients without cancer for most pathogen groups, including vancomycin-resistant enterococci with incidence rate ratio (IRR), 1.95 (95% confidence interval [CI], 1.84, 2.07), extended-spectrum beta-lactamase (ESBL) producers (IRR, 1.48 [95% CI, 1.43, 1.53]), carbapenem-nonsusceptible Enterobacterales (IRR, 1.46 [95% CI, 1.32, 1.61]), and multidrug-resistant Pseudomonas aeruginosa (IRR, 1.31 [95% CI, 1.18, 1.45]). The percentage of nonsusceptible isolates in most pathogen groups was lower in patients with versus without cancer except for ESBL producers among Enterobacterales (odds ratio (OR), 1.11 [95% CI, 1.07, 1.15]) and vancomycin resistance among enterococci (OR, 1.22 [95% CI, 1.14, 1.30]), which were higher in cancer patients.
Conclusion: AMR rates for certain key pathogens were 1.5-2 times greater in hospitalized cancer patients compared to hospitalized noncancer patients. The increased AMR rate in cancer patients highlights the need for enhanced infection prevention and diagnostic stewardship efforts.
Keywords: RRID:SCR_000432; RRID:SCR_001905; antimicrobial resistance (AMR) incidence; hospitalized patients; gram‐negative bacteria; gram‐positive bacteria.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
K.Y., C.A. and D.C.F. are employees of Becton, Dickinson and Company, which was contracted by A.M.R. Action Fund; V.G. was an employee of Becton, Dickinson and Company and owned BD stock at the time of this study. K.Y. and D.C.F. own stock in Becton, Dickinson and Company. K.Y. has/had a leadership or fiduciary role in other board, society, committee, or advocacy groups, as follows: NEST CC (unpaid, FDA initiative to use real‐world data for FDA extended claims), SMI Physicians Committee (unpaid; advising SMI supply chain on how physicians can help mitigate and educate on supply chain issues), and NQF Leadership Consortium (unpaid; NQF LC discusses use cases for unmet needs in healthcare regarding social determinants of health, healthcare provider mental health, etc.). M.J.S. has received grant funding through his institution from Merck, bioMérieux, SNIPRBiome, and Selux Diagnostics, participated on a data and safety monitoring board for AbbVie, and has consulted for Shionogi. L.F.W. has received grant funding from Accelerate Diagnostics Inc., bioMérieux Inc., Hardy Diagnostics, and Roche Molecular Systems Inc., and has consulted for Roche Molecular Systems Inc., Shionogi Inc., and Talis Biomedical. Y.M.M., S.C.H. and L.S. have no conflicts of interest to disclose.
Figures

References
-
- CDC , Antibiotic Resistance Threats in the United States (Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019), 10.15620/cdc:82532 https://stacks.cdc.gov/view/cdc/82532. - DOI
-
- O'Neill J., “Tackling Drug‐Resistant Infections Globally: Final Report and Recommendations,” Government of the United Kingdom (2016), https://amr‐review.org/sites/default/files/160518_Final%20paper_with%20c....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

