Comparison of balloon catheter, oral misoprostol, or combination of both for cervical ripening in late-term and post-term nulliparous women: A Finnish randomized controlled multicenter pilot trial
- PMID: 39673223
- PMCID: PMC11782065
- DOI: 10.1111/aogs.15034
Comparison of balloon catheter, oral misoprostol, or combination of both for cervical ripening in late-term and post-term nulliparous women: A Finnish randomized controlled multicenter pilot trial
Abstract
Introduction: Nulliparous women beyond term have high rates of induction failure. The aim of this study was to compare delivery outcomes for balloon catheter, misoprostol, and combination of both in nulliparous late- and post-term women with unfavorable cervices. We intended to explore whether the combination strategy has lower cesarean section rate and is as safe as either method alone.
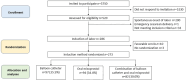
Material and methods: The randomized multicenter pilot trial was carried out in the five university hospitals and the largest secondary care hospital of Finland March 1, 2018-March 31, 2022. A total of 273 nulliparous women with viable singleton fetus in cephalic presentation, intact amniotic membranes, and an unfavorable cervix at 41 + 0 gestational weeks were included. The study protocol was registered in the ISCTN registry (ISRCTN83219789). The women were randomized into cervical ripening by balloon catheter, oral misoprostol 50 μg every 4 h, or the combination use of both. The primary outcomes of the study were the rates of cesarean section and composite of adverse neonatal outcomes (5-min Apgar score <7, umbilical artery pH ≤7.05, or NICU admission).
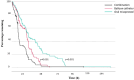
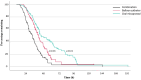
Results: Ninety-seven women (35.5%) were allocated in the balloon catheter arm, 94 (34.4%) in the oral misoprostol arm, and 82 (30.0%) in the combination treatment arm. The rates of cesarean section (balloon catheter 23.7%, n = 23/97, vs. oral misoprostol 24.5%, n = 23/94, vs. combination 17.1%, n = 14/82) or composite adverse neonatal outcome (7.2% vs. 7.4% vs. 7.3%, respectively) were not statistically significantly different between the groups. The median (interquartile range) induction to delivery interval was the shortest in the combination treatment, 21.7 (15.1-33.2) h compared to balloon catheter 31.7 (21.9-44.5) h; p < 0.01 or oral misoprostol 37.0 (26.7-60.3) h; p < 0.01. The proportion of women with induction to delivery interval ≤24 h was significantly higher in the combination arm compared to balloon catheter (54.4% vs. 31.1%; p < 0.01) or oral misoprostol (54.4% vs. 21.1%; p < 0.001).
Conclusions: Our findings confirm the effectiveness of combining balloon catheter and oral misoprostol for cervical ripening, showing shorter induction to delivery interval and comparable rates of cesarean section and neonatal outcomes compared to either method alone. These results advocate for a shift toward adopting combination strategy in the induction of nulliparous late- and post-term women.
Keywords: balloon catheter; cervical ripening; induction of labor; late‐ and post‐term pregnancy; misoprostol; nulliparous women; unfavorable cervix.
© 2024 The Author(s). Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
The authors explicitly state no potential conflicts of interest exist.
Figures
Similar articles
-
Transcervical Foley Balloon Plus Vaginal Misoprostol versus Vaginal Misoprostol Alone for Cervical Ripening in Nulliparous Obese Women: A Multicenter, Randomized, Comparative-Effectiveness Trial.Am J Perinatol. 2021 Aug;38(S 01):e123-e128. doi: 10.1055/s-0040-1708805. Epub 2020 Apr 16. Am J Perinatol. 2021. PMID: 32299108 Clinical Trial.
-
Comparison of balloon catheter and oral misoprostol for cervical ripening in women with pre-labor rupture of membranes: A Finnish randomized controlled trial.Acta Obstet Gynecol Scand. 2025 Feb;104(2):400-407. doi: 10.1111/aogs.15036. Epub 2024 Dec 19. Acta Obstet Gynecol Scand. 2025. PMID: 39697073 Free PMC article. Clinical Trial.
-
Foley Catheter or Oral Misoprostol for Induction of Labor in Women with Term Premature Rupture of Membranes: A Randomized Multicenter Trial.Am J Perinatol. 2016 Jul;33(9):866-72. doi: 10.1055/s-0036-1580608. Epub 2016 Mar 31. Am J Perinatol. 2016. PMID: 27031055 Clinical Trial.
-
Maternal and neonatal outcomes with mechanical cervical dilation plus misoprostol compared to misoprostol alone for cervical ripening; a systematic review of literature and metaanalysis.Am J Obstet Gynecol MFM. 2019 May;1(2):101-111. doi: 10.1016/j.ajogmf.2019.06.003. Epub 2019 Jun 11. Am J Obstet Gynecol MFM. 2019. PMID: 33345815
-
Misoprostol combined with cervical single or double balloon catheters versus misoprostol alone for labor induction of singleton pregnancies: a meta-analysis of randomized trials.J Matern Fetal Neonatal Med. 2020 Oct;33(20):3453-3468. doi: 10.1080/14767058.2019.1574741. Epub 2019 Feb 10. J Matern Fetal Neonatal Med. 2020. PMID: 30741051
References
-
- Australian institute of Health and Welfare (Welfare AIoHa) . Australia's mothers and babies. 2022. https://www.aihw.gov.au/reports/mothers‐babies/australias‐mothers‐babies...
-
- Statista . Number of live births in the European Union (EU27) from 2009 to 2021. 2022. https://www.statista.com/statistics/253401/number‐of‐live‐births‐in‐the‐eu
-
- Hamilton BE, Martin JA, Osterman MJK. Vital Statistics Rapid Release. Births: Provisional Data for 2021. M.H.S., Division of Vital Statistics, National Center for Health Statistics. 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf
-
- Community Health Services, England . NHS Maternity Statistics, England, 2022–23. Official statistics. 2023. https://digital.nhs.uk/data‐and‐information/publications/statistical/nhs...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

