Dupilumab as an Adjunct to Oral Immunotherapy in Pediatric Patients With Peanut Allergy
- PMID: 39673367
- PMCID: PMC11891407
- DOI: 10.1111/all.16420
Dupilumab as an Adjunct to Oral Immunotherapy in Pediatric Patients With Peanut Allergy
Abstract
Background: Peanut allergy is a common, life-threatening food allergy in children. We evaluated whether dupilumab, which blocks the activity of interleukin (IL)-4/IL-13, enhances the efficacy of oral immunotherapy (OIT) AR101 in pediatric patients with peanut allergy.
Methods: A Phase II, multicenter, randomized, double-blind study was conducted in the USA (NCT03682770) in pediatric patients (6-≤ 17 years old) with confirmed peanut allergy. Patients were randomized 2:1 to receive dupilumab + OIT or placebo + OIT during a 28-40-week up-dosing period. Patients in the dupilumab + OIT group were re-randomized 1:1 and received dupilumab + OIT or placebo + OIT during 24-week OIT maintenance, undergoing a 2044 mg (cumulative) of peanut protein double-blind, placebo-controlled food challenge (DBPCFC) following up-dosing, maintenance, and at 12-week post-treatment follow-up.
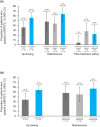
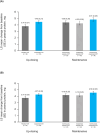
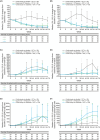
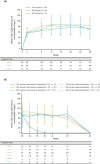
Results: The study enrolled 148 patients, 123 of whom were included in the modified full analysis set, with a mean age of 11.1 years. Dupilumab + OIT treatment (n = 84) led to a 20.2% increase (p < 0.05) in the number of patients who passed a DBPCFC to 2044 mg (cumulative) of peanut protein following the up-dosing period versus placebo (OIT alone, n = 39). Following the OIT maintenance period, continuous dupilumab treatment improved the number of patients who passed a DBPCFC to 2044 mg (cumulative) of peanut protein versus patients continuously on OIT alone (16.6% difference [95% CI -9.7, 42.8], p = 0.2123). Safety was consistent with known dupilumab safety profile.
Conclusions: Dupilumab provided a modest increase efficacy of OIT in children and adolescents with peanut allergy, though it did not provide protection against OIT-related anaphylaxis.
Trial registration: ClinicalTrials.gov identifier: NCT03793608.
Keywords: OIT; children; dupilumab; peanut allergy.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
R.S.C. has received research grants from Aimmune, Astellas, AstraZeneca, Consortium for Food Allergy Research, DBV Technologies, Food Allergy Research & Education, National Institute of Allergy and Infectious Disease, Novartis, Regeneron, and has been an advisory board member for Alladapt, Allergenis, Intrommune Therapeutics, Genentech, Novartis, Sanofi. S.B.S. reports research grants from Aimmune, the Consortium for Food Allergy Research (CoFAR), FARE, DBV Technologies, the National Institute of Allergy and Infectious Diseases (NIAID), Novartis, Regeneron Pharmaceuticals Inc., and Sanofi, and is an advisory board member for DBV Technologies and a consultant for Genentech. K.C.N. has received research grants from Food Allergy Research & Education, National Heart, Lung, and Blood Institute, National Institute of Allergy and Infectious Diseases, National Institute of Environmental Health Sciences, holds stock options in ClostraBio, IgGenix, ImmuneID, Seed Health, is involved in directorship for World Allergy Organization Center of Excellence for Stanford, is an advisor for Cour Pharma, receives consultancy fees from Eli Lilly, Excellergy, Phylaxis, Red Tree Ventures, is a co‐founder of Alladapt, Before Brands, ClostraBio, IgGenix, ImmuneID, Latitude, Seed Health, is a National Scientific Committee member for Immune Tolerance Network, National Institutes of Health clinical research centers, has issued patents (US12/686,121 and US12/610,94), and is a licensee (US15/048,609). J.G.L. has no conflicts to disclose. J.M.S. has received research grants from National Institutes of Health, Regeneron/Sanofi, has received consultancy fees from Allakos, Celgene, Regeneron/Sanofi, and has received royalties from UpToDate. D.H.P. has received speakers' bureau fees from AstraZeneca and is a consultant for HollisterStier. S.M.J. has received grants and/or contracts for the conduct of research and clinical trials from Aimmune Therapeutics, ALK‐Abello Inc., Aravax, PTY, Astellas Pharma Inc., DBV Technologies, Genentech, Novartis, Regeneron, and Food Allergy Research and Education (FARE), NIH‐NIAID, Immune Tolerance Network, and she serves on the board of directors for LAmAb Bio Inc. T.B.C. has received research grants from American Lung Association, Genentech, National Institutes of Health, Novartis, Patient‐Centered Outcomes Research Institute, Sanofi; consultancy fees from AstraZeneca, Boehringer Ingelheim, Genentech, Novartis, Regeneron; and speakers' bureau fees from Genentech, Sanofi. J.W. has received research grants from Aimmune, DBV Technologies, National Institute of Allergy and Infectious Diseases, Regeneron and is a consultant for ALK Abello, Jubilant HollisterStier. WWC has received speaker's fees from AstraZeneca, Boehringer Ingelheim, Regeneron, Sanofi, Teva, and consultancy fees from Aerocrine, Alcon Laboratories, AstraZeneca, Baxalta, Boehringer Ingelheim, Circassia, CSL Behring, Genentech, GlaxoSmithKline, Greer Laboratories, Grifols, Horizon Pharma, Kaleo, Meda, Mylan, Novartis, Pfizer, Regeneron, Sanofi, Shire, Teva, Valeant Pharmaceuticals. WGS has received research grants from National Institutes of Health, Aimmune/NestleAngany, Allergy Therapeutics, Celgene, Food Allergy Science Initiative, Genentech, Novartis, Regeneron; consultancy fees from Aimmune, Food Allergy Research & Education, Novartis; and royalties from UpToDate. R.A.W. has received research grants from Aimmune, DBV Technologies, Food Allergy Research & Education, Genentech, National Institutes of Health, Novartis, Regeneron, Siolta, and royalties from UpToDate. E.W. has received grant support from Aimmune, AnaptysBio, Astellas, Cour Pharma, Food Allergy Research & Education, Immune Tolerance Network, National Institute of Allergy and Infectious Disease, and research sponsorship from Regeneron. J.L., B.A., M.A.K., A.A., and A.R.R. are employees of, and may hold stock and/or stock options in, Regeneron Pharmaceuticals Inc. J.D.H. is an employee of, and may hold stock and/or stock options in, Regeneron Pharmaceuticals Inc. and has a patent pending (PCT/US2020/044958) with Regeneron Pharmaceuticals Inc. J.M.O. is an officer and employee at Regeneron Pharmaceuticals Inc., may hold stock and/or stock options, has a patent pending (PCT/US2020/0345843, PCT/US2022/0220211), and has issued patents (US patent numbers 10,392,439, 10,676,530, and 11,485,788). K.P., E.L., and L.P.M. are employees of, and may hold stock and/or stock options in, Sanofi. A.T.H. is an employee of, and may hold stock and/or stock options in, Regeneron Pharmaceuticals Inc. D.C.A. has received consultancy fees from Aimmune and has a patent pending (US 16/542,198; PCT/US2019/046706; US 16/721,805; PCT/US2019/067634). A.R. is an employee of, and may hold stock and/or stock options in, Aimmune.
Figures

References
-
- Dyer A. A. and Gupta R., “Epidemiology of Childhood Food Allergy,” Pediatric Annals 42, no. 6 (2013): 91–95. - PubMed

