Real-World Effectiveness of Tildrakizumab for Moderate-to-Severe Plaque Psoriasis in Canada
- PMID: 39673433
- PMCID: PMC11979299
- DOI: 10.1177/12034754241302827
Real-World Effectiveness of Tildrakizumab for Moderate-to-Severe Plaque Psoriasis in Canada
Abstract
Background: Tildrakizumab is an interleukin-23 inhibitor approved in Canada in 2021 for the treatment of adults with moderate-to-severe plaque psoriasis.
Objectives: To evaluate real-world effectiveness of tildrakizumab for the treatment of moderate-to-severe plaque psoriasis in Canada.
Methods: A multicenter, retrospective study was conducted in Canada in adults with moderate-to-severe plaque psoriasis for ≥1 year treated with tildrakizumab for ≥12 weeks. Effectiveness was evaluated from proportions of patients achieving ≥75%/≥90%/100% improvement from baseline in Psoriasis Area and Severity Index (PASI 75/90/100 response) and Physician Global Assessment (PGA) 0 or 1 at weeks 16 (±4), 24 (±8), and 48 (±12). Subgroup analyses were performed based on prior biologic use and special site involvement.
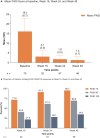
Results: The study included 75 patients (mean age, 50.5 years; 52.0% female; 82.7% bio-naïve; 73.3% with special site involvement). Absolute mean (standard deviation) PASI score improved from 16.1 (6.7) at baseline to 1.3 (1.7) at the week 48 (91.7% improvement), 95.7%/69.6%/34.8% of patients achieved PASI 75/90/100 response, and 93.0% achieved PGA 0/1 at the week 48. In subgroup analyses, 94.7%/71.1%/34.2% of bio-naïve patients, 100.0%/62.5%/37.5% of bio-experienced patients, 100.0%/71.4%/28.6% of patients with special site involvement, and 81.8%/63.6%/54.6% of patients without special site involvement achieved PASI 75/90/100 response, and 87.5%, 94.3%, 97.0%, and 80.0% of patients, respectively, achieved PGA 0/1 at the week 48. None of the differences among subgroups were statistically significant; however, patient numbers were too small to support robust conclusions.
Conclusions: Tildrakizumab is effective for the treatment of moderate-to-severe plaque psoriasis in adults in a real-world setting in Canada.
Keywords: anti–IL-23; psoriasis; real-world; tildrakizumab.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MAH received advisory board honoraria and consulting fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galderma, Hikma Pharmaceuticals, Janssen, LEO Pharma, L’Oréal, Medexus Pharma, Novartis, Pfizer, Sanofi, and Sun Pharma. AC and JC have served on advisory boards, received honoraria, or contributed to clinical trials for AbbVie, Amgen, Aralez Pharmaceuticals, Bausch Health, Celgene Corporation, Eli Lilly, Galderma, Janssen, Johnson and Johnson, LEO Pharma, Novartis, Pfizer, Sanofi, UCB, and Valeant. MB, DS, and IS have no conflicts of interest to declare. BY, RES, and TB are employees of Cencora, Innomar Strategies, Inc. PC is an employee of Sun Pharma Canada Inc.
Figures
References
-
- Menter A, Gottlieb A, Feldman SR, et al.. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-850. doi:10.1016/j.jaad.2008.02.039 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical